Manufacturing Establishments (1)
Amneal Pharmaceuticals NY LLC
123797875
Products (1)
Sildenafil
69238-1574
ANDA211092
ANDA (C73584)
ORAL
August 18, 2023
Drug Labeling Information
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Sildenafil is an inhibitor of cGMP specific PDE-5 in the smooth muscle of the pulmonary vasculature, where PDE-5 is responsible for degradation of cGMP. Sildenafil, therefore, increases cGMP within pulmonary vascular smooth muscle cells resulting in relaxation. In patients with PAH, this can lead to vasodilation of the pulmonary vascular bed and, to a lesser degree, vasodilatation in the systemic circulation.
Studies in vitro have shown that sildenafil is selective for PDE-5. Its effect is more potent on PDE-5 than on other known phosphodiesterases (10-fold for PDE6, greater than 80-fold for PDE1, greater than 700-fold for PDE2, PDE3, PDE4, PDE7, PDE8, PDE9, PDE10, and PDE11). The approximately 4,000-fold selectivity for PDE-5 versus PDE3 is important because PDE3 is involved in control of cardiac contractility. Sildenafil is only about 10 times as potent for PDE5 compared to PDE6, an enzyme found in the retina and involved in the phototransduction pathway of the retina. This lower selectivity is thought to be the basis for abnormalities related to color vision observed with higher doses or plasma levels [see Clinical Pharmacology (12.2)].
In addition to pulmonary vascular smooth muscle and the corpus cavernosum, PDE5 is also found in other tissues including vascular and visceral smooth muscle and in platelets. The inhibition of PDE5 in these tissues by sildenafil may be the basis for the enhanced platelet anti-aggregatory activity of nitric oxide observed in vitro, and the mild peripheral arterial-venous dilatation in vivo.
12.2 Pharmacodynamics
Effects of Sildenafil on Hemodynamic Measures
Adults
Patients on all sildenafil doses achieved a statistically significant reduction in mean pulmonary arterial pressure (mPAP) compared to those on placebo in a study with no background vasodilators [see SUPER-1 in Clinical Studies (14)]. Data on other hemodynamic measures for the sildenafil 20 mg three times a day and placebo dosing regimens is displayed in Table 2. The relationship between these effects and improvements in 6-minute walk distance is unknown.
Table 2: Changes from Baseline in Hemodynamic Parameters at Week 12 [mean (95% CI)] for the Sildenafil 20 mg Three Times a Day and Placebo Group
|
Placebo (n = 65)***** |
Sildenafil 20 mg (n = 65)***** | |
|
mPAP (mmHg) |
0.6 (-0.8, 2) |
-2.1 (-4.3, 0) |
|
PVR (dyn•s/cm5) |
49 (-54, 153) |
-122 (-217, -27) |
|
SVR (dyn•s/cm5) |
-78 (-197, 41) |
-167 (-307, -26) |
|
RAP (mmHg) |
0.3 (-0.9, 1.5) |
-0.8 (-1.9, 0.3) |
|
CO (L/min) |
-0.1 (-0.4, 0.2) |
0.4 (0.1, 0.7) |
|
HR (beats/min) |
-1.3 (-4.1, 1.4) |
-3.7 (-5.9, -1.4) |
|
mPAP = mean pulmonary arterial pressure; PVR = pulmonary vascular resistance; SVR = systemic vascular resistance; RAP = right atrial pressure; CO = cardiac output; HR = heart rate. *The number of patients per treatment group varied slightly for each parameter due to missing assessments. |
Effects of Sildenafil on Blood Pressure
Single oral doses of sildenafil 100 mg administered to healthy volunteers produced decreases in supine blood pressure (mean maximum decrease in systolic/diastolic blood pressure of 8/5 mmHg). The decrease in blood pressure was most notable approximately 1 hour to 2 hours after dosing and was not different from placebo at 8 hours. Similar effects on blood pressure were noted with 25 mg, 50 mg, and 100 mg doses of sildenafil, therefore the effects are not related to dose or plasma levels within this dosage range. Larger effects were recorded among patients receiving concomitant nitrates [see Contraindications (4)].
Single oral doses of sildenafil up to 100 mg in healthy volunteers produced no clinically relevant effects on electrocardiogram (ECG). After chronic dosing of 80 mg three times a day to patients with PAH, no clinically relevant effects on ECG were reported.
After chronic dosing of 80 mg three times a day sildenafil to healthy volunteers, the largest mean change from baseline in supine systolic and supine diastolic blood pressures was a decrease of 9 mmHg and 8.4 mmHg, respectively.
After chronic dosing of 80 mg three times a day sildenafil to patients with systemic hypertension, the mean change from baseline in systolic and diastolic blood pressures was a decrease of 9.4 mmHg and 9.1 mmHg, respectively.
After chronic dosing of 80 mg three times a day sildenafil to patients with PAH, lesser reductions than above in systolic and diastolic blood pressures were observed (a decrease in both of 2 mmHg).
Effects of Sildenafil on Vision
At single oral doses of 100 mg and 200 mg, transient dose-related impairment of color discrimination (blue/green) was detected using the Farnsworth-Munsell 100-hue test, with peak effects near the time of peak plasma levels. This finding is consistent with the inhibition of PDE6, which is involved in phototransduction in the retina. An evaluation of visual function at doses up to 200 mg revealed no effects of sildenafil on visual acuity, intraocular pressure, or pupillometry.
Pediatric use information is approved for Viatris Specialty LLC’s, REVATIO (sildenafil) oral suspension. However, due to Viatris Specialty LLC’s marketing exclusivity rights, this drug product is not labeled with that information.
12.3 Pharmacokinetics
Absorption and Distribution
Sildenafil is rapidly absorbed after oral administration, with a mean absolute bioavailability of 41% (25% to 63%). Maximum observed plasma concentrations are reached within 30 minutes to 120 minutes (median 60 minutes) of oral dosing in the fasted state. When sildenafil is taken with a high-fat meal, the rate of absorption is reduced, with a mean delay in Tmax of 60 minutes and a mean reduction in Cmax of 29%. The mean steady-state volume of distribution (Vss) for sildenafil is 105 L, indicating distribution into the tissues. Sildenafil and its major circulating N-desmethyl metabolite are both approximately 96% bound to plasma proteins. Protein binding is independent of total drug concentrations.
Bioequivalence was established between the 20 mg tablet and the 10 mg/mL oral suspension when administered as a 20 mg single oral dose of sildenafil (as citrate).
Metabolism and Excretion
Sildenafil is cleared predominantly by the CYP3A (major route) and cytochrome P450 2C9 (CYP2C9, minor route) hepatic microsomal isoenzymes. The major circulating metabolite results from N-desmethylation of sildenafil, and is, itself, further metabolized. This metabolite has a phosphodiesterase selectivity profile similar to sildenafil and an in vitro potency for PDE-5 approximately 50% of the parent drug. In healthy volunteers, plasma concentrations of this metabolite are approximately 40% of those seen for sildenafil, so that the metabolite accounts for about 20% of sildenafil’s pharmacologic effects. In patients with PAH, however, the ratio of the metabolite to sildenafil is higher. Both sildenafil and the active metabolite have terminal half-lives of about 4 hours.
After oral administration, sildenafil is excreted as metabolites predominantly in the feces (approximately 80% of the administered oral dose) and to a lesser extent in the urine (approximately 13% of the administered oral dose).
Population Pharmacokinetics
Age, gender, race, and renal and hepatic function were included as factors assessed in the population pharmacokinetic model to evaluate sildenafil pharmacokinetics in patients with PAH. The dataset available for the population pharmacokinetic evaluation contained a wide range of demographic data and laboratory parameters associated with hepatic and renal function. None of these factors had a significant impact on sildenafil pharmacokinetics in patients with PAH.
In patients with PAH, the average steady-state concentrations were 20% to 50% higher when compared to those of healthy volunteers. There was also a doubling of Cmin levels compared to healthy volunteers. Both findings suggest a lower clearance and/or a higher oral bioavailability of sildenafil in patients with PAH compared to healthy volunteers.
Pediatric Patients
Pediatric use information is approved for Viatris Specialty LLC’s, REVATIO (sildenafil) oral suspension. However, due to Viatris Specialty LLC’s marketing exclusivity rights, this drug product is not labeled with that information.
Geriatric Patients
Healthy elderly volunteers (65 years or over) had a reduced clearance of sildenafil, resulting in approximately 84% and 107% higher plasma concentrations of sildenafil and its active N-desmethyl metabolite, respectively, compared to those seen in healthy younger volunteers (18 years to 45 years). Due to age-differences in plasma protein binding, the corresponding increase in the AUC of free (unbound) sildenafil and its active N-desmethyl metabolite were 45% and 57%, respectively.
Renal Impairment
In volunteers with mild (CLcr = 50 mL/min to 80 mL/min) and moderate (CLcr = 30 mL/min to 49 mL/min) renal impairment, the pharmacokinetics of a single oral dose of sildenafil (50 mg) was not altered. In volunteers with severe (CLcr less than 30 mL/min) renal impairment, sildenafil clearance was reduced, resulting in approximately doubling of AUC and Cmax compared to age-matched volunteers with no renal impairment. In addition, N-desmethyl metabolite AUC and Cmax values were significantly increased 200% and 79%, respectively, in patients with severe renal impairment compared to patients with normal renal function.
Hepatic Impairment
In volunteers with mild to moderate hepatic cirrhosis (Child-Pugh class A and B), sildenafil clearance was reduced, resulting in increases in AUC (84%) and Cmax (47%) compared to age-matched volunteers with no hepatic impairment. Patients with severe hepatic impairment (Child-Pugh class C) have not been studied.
Drug Interaction Studies
In vitro studies
Sildenafil metabolism is principally mediated by the CYP3A (major route) and CYP2C9 (minor route) cytochrome P450 isoforms. Therefore, inhibitors of these isoenzymes may reduce sildenafil clearance and inducers of these isoenzymes may increase sildenafil clearance.
Sildenafil is a weak inhibitor of the cytochrome P450 isoforms 1A2, 2C9, 2C19, 2D6, 2E1 and 3A (IC50 greater than 150 μM).
Sildenafil is not expected to affect the pharmacokinetics of compounds which are substrates of these CYP enzymes at clinically relevant concentrations.
In vivo studies
The effects of other drugs on sildenafil pharmacokinetics and the effects of sildenafil on the exposure to other drugs are shown in Figure 1 and Figure 2, respectively.
Figure 1: Effects of Other Drugs on Sildenafil Pharmacokinetics
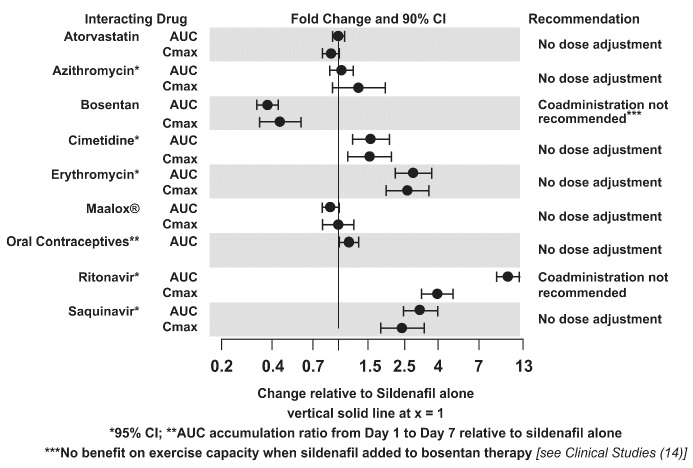
Figure 2: Effects of Sildenafil on Other Drugs
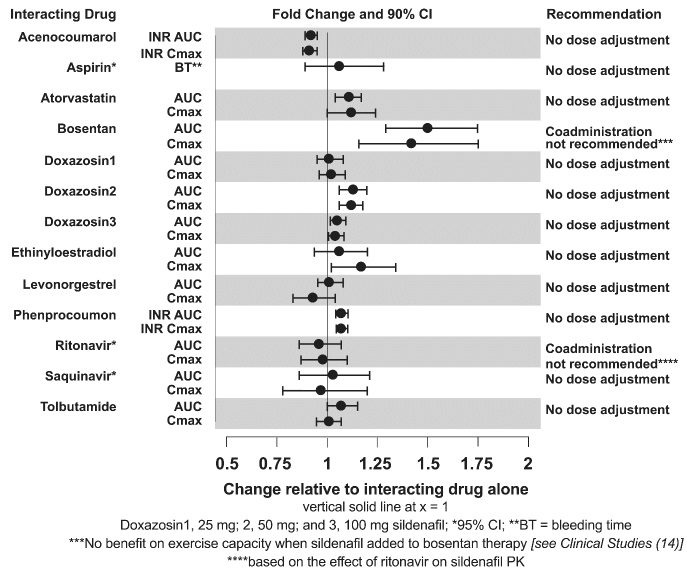
CYP3A Inhibitors and Beta Blockers
Population pharmacokinetic analysis of data from patients in clinical trials indicated an approximately 30% reduction in sildenafil clearance when it was co-administered with mild/moderate CYP3A inhibitors and an approximately 34% reductions in sildenafil clearance when co-administered with beta-blockers. Sildenafil exposure at a dose of 80 mg three times a day without concomitant medication is shown to be 5-fold the exposure at a dose of 20 mg three times a day. This concentration range covers the same increased sildenafil exposure observed in specifically-designed drug interaction studies with CYP3A inhibitors (except for potent inhibitors such as ketoconazole, itraconazole, and ritonavir).
CYP3A4 Inducers Including Bosentan
Concomitant administration of strong CYP3A inducers is expected to cause substantial decreases in plasma levels of sildenafil.
Population pharmacokinetic analysis of data from patients in clinical trials indicated approximately 3-fold the sildenafil clearance when it was co- administered with mild CYP3A inducers.
Epoprostenol
The mean reduction of sildenafil (80 mg three times a day) bioavailability when co-administered with epoprostenol was 28%, resulting in about 22% lower mean average steady-state concentrations. Therefore, the slight decrease of sildenafil exposure in the presence of epoprostenol is not considered clinically relevant. The effect of sildenafil on epoprostenol pharmacokinetics is not known.
No significant interactions were shown with tolbutamide (250 mg) or warfarin (40 mg), both of which are metabolized by CYP2C9.
Alcohol
Sildenafil (50 mg) did not potentiate the hypotensive effect of alcohol in healthy volunteers with mean maximum blood alcohol levels of 0.08%.
INDICATIONS & USAGE SECTION
Highlight: Adults
Sildenafil for oral suspension is a phosphodiesterase-5 (PDE-5) inhibitor indicated for the treatment of pulmonary arterial hypertension (PAH) (World Health Organization [WHO] Group I) in adults to improve exercise ability and delay clinical worsening. (1)
1 INDICATIONS AND USAGE
Adults
Sildenafil for oral suspension is indicated for the treatment of pulmonary arterial hypertension (PAH) (World Health Organization [WHO] Group I) in adults to improve exercise ability and delay clinical worsening [see Clinical Studies (14)].
Pediatric use information is approved for Viatris Specialty LLC’s, REVATIO (sildenafil) oral suspension. However, due to Viatris Specialty LLC’s marketing exclusivity rights, this drug product is not labeled with that information.
DOSAGE & ADMINISTRATION SECTION
Highlight: * Adults: 20 mg three times a day. (2.1)
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage in Adults
Oral Dosage
The recommended dosage of sildenafil for oral suspension is 20 mg three times a day [see Clinical Studies (14)].
Pediatric use information is approved for Viatris Specialty LLC’s, REVATIO (sildenafil) oral suspension. However, due to Viatris Specialty LLC’s marketing exclusivity rights, this drug product is not labeled with that information.
2.3 Reconstitution of the Powder for Oral Suspension
Note: Reconstitute the contents of the bottle with a total volume of 90 mL (60 mL followed by 30 mL). Refer to the detailed instructions below.
- Tap the bottle to loosen the powder.
- Add 60 mL of water to the bottle.
- Replace the cap and shake the bottle vigorously for a minimum of 30 seconds.
- Add another 30 mL of water to the bottle.
- Replace the cap and shake the bottle vigorously for a minimum of 30 seconds.
- Remove the cap and press the bottle adaptor into the neck of the bottle. Replace the cap on the bottle.
- Write the expiration date of the reconstituted oral suspension on the bottle label (the expiration date of the reconstituted oral suspension is 60 days from the date of reconstitution).
Incompatibilities
Do not mix with any other medication or additional flavoring agent.
DRUG INTERACTIONS SECTION
Highlight: * Use with strong CYP3A inhibitors: Not recommended. (7, 12.3)
- Concomitant PDE-5 inhibitors: Avoid use with Viagra® or other PDE-5 inhibitors. (5.6)
Pediatric use information is approved for Viatris Specialty LLC’s, REVATIO (sildenafil) oral suspension. However, due to Viatris Specialty LLC’s marketing exclusivity rights, this drug product is not labeled with that information.
7 DRUG INTERACTIONS
Nitrates
Concomitant use of sildenafil with nitrates in any form is contraindicated [see Contraindications (4)].
Strong CYP3A Inhibitors
Concomitant use of sildenafil with strong CYP3A inhibitors is not recommended [see Clinical Pharmacology (12.3)].
Moderate-to-Strong CYP3A Inducers
Concomitant use of sildenafil with moderate-to-strong CYP3A inducers (such as bosentan) decreases the sildenafil exposure. Dose up-titration of sildenafil may be needed when initiating treatment with moderate-to-strong CYP3A inducers. Reduce the dose of sildenafil to 20 mg three times a day when discontinuing treatment with moderate-to-strong CYP3A inducers [see Clinical Pharmacology (12.3) and Clinical Studies (14)].
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Limited published data from randomized controlled trials, case-controlled trials, and case series do not report a clear association with sildenafil and major birth defects, miscarriage, or adverse maternal or fetal outcomes when sildenafil is used during pregnancy. There are risks to the mother and fetus from untreated pulmonary arterial hypertension (see Clinical Considerations). Animal reproduction studies conducted with sildenafil showed no evidence of embryo-fetal toxicity or teratogenicity at doses up to 32- and 65-times the recommended human dose (RHD) of 20 mg three times a day in rats and rabbits, respectively (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
Pregnant women with untreated pulmonary arterial hypertension are at risk for heart failure, stroke, preterm delivery, and maternal and fetal death.
Data
Animal Data
No evidence of teratogenicity, embryotoxicity, or fetotoxicity was observed in pregnant rats or rabbits dosed with sildenafil 200 mg/kg/day during organogenesis, a level that is, on a mg/m2 basis, 32- and 65-times, respectively, the recommended human dose (RHD) of 20 mg three times a day. In a rat pre- and postnatal development study, the no-observed-adverse-effect dose was 30 mg/kg/day (equivalent to 5-times the RHD on a mg/m2 basis).
8.2 Lactation
Risk Summary
Limited published data from a case report describe the presence of sildenafil and its active metabolite in human milk. There is insufficient information about the effects of sildenafil on the breastfed infant and no information on the effects of sildenafil on milk production. Limited clinical data during lactation preclude a clear determination of the risk of sildenafil to an infant during lactation.
8.4 Pediatric Use
The safety and effectiveness of sildenafil has not been established in pediatric patients younger than 1 year of age.
Pediatric use information is approved for Viatris Specialty LLC’s, REVATIO (sildenafil) oral suspension. However, due to Viatris Specialty LLC’s marketing exclusivity rights, this drug product is not labeled with that information.
8.5 Geriatric Use
Clinical studies of sildenafil did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy [see Clinical Pharmacology (12.3)].
8.6 Patients with Hepatic Impairment
No dose adjustment for mild to moderate impairment is required. Severe impairment has not been studied [see Clinical Pharmacology (12.3)].
8.7 Patients with Renal Impairment
No dose adjustment is required (including severe impairment CLcr < 30 mL/min) [see Clinical Pharmacology (12.3)].
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Sildenafil was not carcinogenic when administered to rats for up to 24 months at 60 mg/kg/day, a dose resulting in total systemic exposure (AUC) to unbound sildenafil and its major metabolite 33- and 37-times, for male and female rats, respectively, the human exposure at the RHD of 20 mg three times a day. Sildenafil was not carcinogenic when administered to male and female mice for up to 21 months and 18 months, respectively, at doses up to a maximally tolerated level of 10 mg/kg/day, a dose equivalent to the RHD on a mg/m2 basis.
Sildenafil was negative in in vitro bacterial and Chinese hamster ovary cell assays to detect mutagenicity, and in vitro human lymphocytes and in vivo mouse micronucleus assays to detect clastogenicity.
There was no impairment of fertility in male or female rats given up to 60 mg sildenafil/kg/day, a dose producing a total systemic exposure (AUC) to unbound sildenafil and its major metabolite of 19- and 38-times for males and females, respectively, the human exposure at the RHD of 20 mg three times a day.
SPL PATIENT PACKAGE INSERT SECTION
PATIENT INFORMATION
|
Sildenafil**(sil den’ a fil)**for Oral Suspension | |
|
What is the most important information I should know about sildenafil for oral suspension? Never take sildenafil for oral suspension with any nitrate or guanylate cyclase stimulator medicines.
Nitrates include:
Guanylate cyclase stimulators include:
Ask your healthcare provider or pharmacist if you are not sure if you are taking a nitrate or a guanylate cyclase stimulator medicine. See**“What are the possible side effects of sildenafil for oral suspension?”** for more information about side effects. | |
|
What is sildenafil for oral suspension? Sildenafil for oral suspension is a prescription medicine used to treat pulmonary arterial hypertension (PAH). PAH is a type of high blood pressure in the arteries of your lungs. Sildenafil for oral suspension may be used in:
It is not known if sildenafil for oral suspension is safe and effective in children younger than 1 year of age. | |
|
Do not take sildenafil for oral suspension if you:
| |
|
Before taking sildenafil for oral suspension tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all of the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Sildenafil for oral suspension and certain other medicines may affect each other and can cause side effects. Especially tell your healthcare provider if you take:
Ask your healthcare provider or pharmacist for a list of these medicines if you are not sure. Know the medicines you take. Keep a list of your medicines and show it to your healthcare provider and pharmacist when you get a new medicine. | |
|
How should I take sildenafil for oral suspension?
| |
|
What are the possible side effects of sildenafil for oral suspension? Sildenafil for oral suspension may cause serious side effects, including:
The most common side effects of sildenafil for oral suspension****in adults include: | |
|
|
|
|
|
|
|
|
|
These are not all the possible side effects of sildenafil for oral suspension. Call your doctor for medical advice about side effects. You may report side effects to Amneal Pharmaceuticals at 1-877-835-5472 or FDA at 1-800-FDA-1088. | |
|
How should I store sildenafil for oral suspension?
Keep sildenafil for oral suspension and all medicines out of the reach of children. | |
|
General information about the safe and effective use of sildenafil for oral suspension Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use sildenafil for oral suspension for a condition for which it was not prescribed. Do not give sildenafil for oral suspension to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about sildenafil for oral suspension that is written for health professionals. | |
|
What are the ingredients in sildenafil for oral suspension? Sildenafil for oral suspension Active ingredients: sildenafil citrate, USP Inactive ingredients: anhydrous citric acid, colloidal silicon dioxide, grape flavor, sodium benzoate, sodium citrate dihydrate, sorbitol, sucralose, titanium dioxide, and xanthan gum For more information go to www.amneal.com or call 1-877-835-5472. This Patient Information has been approved by the U.S. Food and Drug Administration. Trademarks are the property of their respective owner. Pediatric use information is approved for Viatris Specialty LLC’s, REVATIO (sildenafil) oral suspension. However, due to Viatris Specialty LLC’s marketing exclusivity rights, this drug product is not labeled with that information. Distributed by: Amneal Pharmaceuticals LLC Bridgewater, NJ 08807 Rev. 11-2023-04 |
