Kyzatrex
These highlights do not include all the information needed to use KYZATREX™ safely and effectively. See full prescribing information for KYZATREX™. KYZATREX™ (testosterone undecanoate) capsules, for oral use, CIII Initial U.S. approval: 1953
7f7167a7-2a25-47e2-acf5-33f499fce971
HUMAN PRESCRIPTION DRUG LABEL
Aug 13, 2025
Marius Pharmaceuticals
DUNS: 080572348
Products 3
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
TESTOSTERONE UNDECANOATE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
TESTOSTERONE UNDECANOATE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
TESTOSTERONE UNDECANOATE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL




INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
KYZATREX is indicated for testosterone replacement therapy in adult males for conditions associated with a deficiency or absence of endogenous testosterone:
- Primary hypogonadism (congenital or acquired): testicular failure due to conditions such as cryptorchidism, bilateral torsion, orchitis, vanishing testis syndrome, orchiectomy, Klinefelter syndrome, chemotherapy, or toxic damage from alcohol or heavy metals. These men usually have low serum testosterone concentrations and gonadotropins (folliclestimulating hormone (FSH), luteinizing hormone (LH)) above the normal range.
- Hypogonadotropic hypogonadism (congenital or acquired): gonadotropin or luteinizing hormone-releasing hormone (LHRH) deficiency, pituitary-hypothalamic injury from tumors, trauma, or radiation. These men have low serum testosterone concentrations but have gonadotropins in the normal or low range.
Limitations of Use
Safety and efficacy of KYZATREX in males less than 18 years old have not been established [see Use in Specific Populations (8.4)] .
Safety and efficacy of KYZATREX in men with “age-related hypogonadism” (also referred to as “late-onset hypogonadism”) have not been established.
KYZATREX is an androgen indicated for testosterone replacement therapy in adult males for conditions associated with a deficiency or absence of endogenous testosterone ( 1).
Limitations of Use:
- Safety and efficacy of KYZATREX in males less than 18 years old have not been established ( 1, 8.4).
- Safety and efficacy of KYZATREX in men with “age-related hypogonadism” have not been established (1).
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
KYZATREX™ is contraindicated in:
- Patients with carcinoma of the breast or known or suspected carcinoma of the prostate [see Warnings and Precautions (5.34)] .
- Women who are pregnant. Testosterone can cause virilization of the female fetus when administered to a pregnant woman [ see Use in Specific Populations (8.1)].
- Patients with known hypersensitivity to KYZATREX™ or any of its ingredients [see Description (11)] .
- Carcinoma of the breast or known or suspected carcinoma of the prostate ( 5.4)
- Women who are pregnant. Testosterone may cause fetal harm ( 4, 5.7, 8.1)
- Hypersensitivity to KYZATREX™ or any of its ingredients ( 4)
- Hypogonadal conditions not associated with structural or genetic etiologies ( 4)
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Polycythemia
Androgens, including KYZATREX, can cause increase in hemoglobin or hematocrit, reflective of increase in red blood cell mass. Check hematocrit prior to initiating KYZATREX. An increase in red blood cell mass may increase the risk of thromboembolic events [see Warnings and Precautions ( 5.2)]. Evaluate hematocrit approximately every 3 months while the patient is on KYZATREX. If hematocrit becomes elevated, stop KYZATREX until the hematocrit decreases to an acceptable concentration. If KYZATREX is restarted and again causes hematocrit to become elevated, permanently discontinue KYZATREX.
5.2 Venous Thromboembolism
There have been post-marketing reports of venous thromboembolic events, including deep vein thrombosis (DVT) and pulmonary embolism (PE), in patients using testosterone replacement products such as KYZATREX.
In the Testosterone Replacement therapy for Assessment of long-term Vascular Events and efficacy Response in hypogonadal men (TRAVERSE) Study, a randomized, double-blind, placebo-controlled, cardiovascular (CV) outcomes study, compared to placebo, topical testosterone gel was associated with a numerically higher incidence of VTE (1.7% vs 1.2%) which included DVT (0.6% vs 0.5%) and PE events (0.9% vs 0.5%) [see Adverse Reactions 6.1)].
Evaluate patients who report symptoms of pain, edema, warmth, and erythema in the lower extremity for DVT and those who present with acute shortness of breath for PE. If a venous thromboembolic event is suspected, discontinue KYZATREX and initiate appropriate workup and management [see Adverse Reactions ( 6.2)].
5.3 Worsening of Benign Prostatic Hyperplasia (BPH) and Potential Risk of
Prostate Cancer
- Patients with BPH who are treated with androgens are at an increased risk for worsening of signs and symptoms of BPH. Monitor patients with BPH for worsening signs and symptoms.
- Patients treated with androgens may be at increased risk for prostate cancer. Evaluate patients for prostate cancer prior to initiating and during treatment with androgens [see Contraindications (4)].
5.4 Blood Pressure Increases
KYZATREX can increase blood pressure. Based on ambulatory blood monitoring in Study MRS-TU-2019EXT, KYZATREX increased mean systolic/diastolic blood pressure by 1.7/0.6 mm Hg from baseline after 4 months of treatment and 1.8/0.6 mm Hg from baseline after 6 months of treatment [see Adverse Reactions (6.1)]. In patients with hypertension on antihypertensive therapy, KYZATREX increased the mean systolic/diastolic BP by 3.4/0.7 mm Hg from baseline after 4 months of treatment and 3.1/1.0 mm Hg from baseline after 6 months of treatment. [see Adverse Reactions (6.1)]. Blood pressure increases can increase cardiovascular (CV) risk over time.
The CV risk associated with topical testosterone gel was evaluated in TRAVERSE, a randomized, double-blind, placebo controlled, CV outcomes study in men with a history of CV disease or multiple CV risk factors. In TRAVERSE, topical testosterone gel increased mean systolic blood pressure by 1.0 mm Hg from baseline to 36 months, whereas a mean decrease from baseline of 0.5 mm Hg. However, the incidences of major adverse cardiovascular events (MACE), including cardiovascular death, non-fatal myocardial infarction [MI] and non- fatal stroke, were similar between treatment groups (7% for topical testosterone gel vs 7.3% for placebo) [See Adverse Reactions (6.1)]
Monitor BP periodically in men using KYZATREX, especially men with hypertension. KYZATREX is not recommended for use in patients with uncontrolled hypertension.
5.5 Abuse of Testosterone and Monitoring of Testosterone Centrations.
Testosterone has been subject to abuse, typically at doses higher than recommended for the approved indication and in combination with other anabolic androgenic steroids. Anabolic androgenic steroid abuse can lead to serious cardiovascular and psychiatric adverse reactions [see Drug Abuse and Dependence (9)].
If testosterone abuse is suspected, check testosterone concentrations to ensure they are within therapeutic range [see Dosage and Administration ( 2.2)]. Testosterone levels may remain in the normal or subnormal range in men abusing synthetic testosterone derivatives. Counsel patients concerning the serious adverse reactions associated with abuse of testosterone and anabolic androgenic steroids. Also consider the possibility of testosterone and anabolic androgenic steroid abuse in suspected patients who present with serious cardiovascular or psychiatric adverse events.
5.6 Not for Use In Women
Due to lack of controlled studies in women and potential virilizing effects, KYZATREX is not indicated for use in women [see Contraindications (4) and Use in Specific Populations (8.1, 8.2)].
5.7 Potential for Adverse Effects on Spermatogenesis
With large doses of exogenous androgens, including KYZATREX, spermatogenesis may be suppressed through feedback inhibition of pituitary FSH, possibly leading to adverse effects on semen parameters including sperm count [see Use in Specific Populations (8.3)]. Inform patients of this possible risk when deciding whether to use or to continue to use KYZATREX.
5.8 Hepatic Adverse Effects
KYZATREX is not a 17-alpha-alkyl androgen and is not known to cause hepatic adverse effects. However, prolonged use of high doses of orally active 17-alpha-alkyl androgens (e.g., methyltestosterone) has been associated with serious hepatic adverse effects (peliosis hepatis, hepatic neoplasms, cholestatic hepatitis, and jaundice). Peliosis hepatis can be a life- threatening or fatal complication. Long-term therapy with intramuscular testosterone enanthate has produced multiple hepatic adenomas. Patients should be instructed to report any signs or symptoms of hepatic dysfunction (e.g., jaundice). If these occur, promptly discontinue KYZATREX while the cause is evaluated.
5.9 Edema
Androgens, including KYZATREX, may promote retention of sodium and water. Edema, with or without congestive heart failure, may be a serious complication in patients with pre-existing cardiac, renal, or hepatic disease [see Adverse Reactions (6.1)]. In addition to discontinuation of the drug, diuretic therapy may be required.
5.10 Sleep Apnea
The treatment of hypogonadal men with testosterone may potentiate sleep apnea in some patients, especially those with risk factors such as obesity or chronic lung disease.
5.11 Gynecomastia
Gynecomastia may develop and persist in patients being treated for hypogonadism.
5.12 Lipid Changes
In clinical trials, patients receiving KYZATREX experienced reductions in lipid parameters, including total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides [see Adverse Reactions (6.1)]. Changes in the serum lipid profile may require dose adjustment of lipid lowering drugs or discontinuation of testosterone therapy. Monitor the lipid profile periodically, particularly after starting testosterone therapy.
5.13 Hypercalcemia
Androgens, including KYZATREX, should be used with caution in cancer patients at risk of hypercalcemia (and associated hypercalciuria). Monitor serum calcium concentrations periodically during treatment with KYZATREX in these patients.
5.14 Descreased Thyroxine-binding Globulin
Androgens, including KYZATREX, may decrease concentrations of thyroxin-binding globulin, resulting in decreased total T4 serum concentrations and increased resin uptake of T3 and T4. Free thyroid hormone concentrations remain unchanged, however, and there is no clinical evidence of thyroid dysfunction.
- Polycythemia: Monitor hemoglobin or hematocrit approximately every 3 months to detect increased red blood cell mass and polycythemia. Discontinue KYZATREX™ if necessary ( 5.1).
- Venous thromboembolism (VTE): VTE, including deep vein thrombosis (DVT) and pulmonary embolism (PE) have been reported in patients using testosterone. Discontinue KYZATREX if VTE is suspected and initiate appropriate workup and management (5.2)
- Worsening of Benign Prostatic Hyperplasia (BPH) and Potential Risk of Prostate Cancer: Monitor patients for worsening of signs and symptoms of BPH. Evaluate patients for prostate cancer, including monitoring prostate specific antigen (PSA) prior to initiating and during treatment with androgens ( 5.3).
- Blood Pressure Increases: KYZATREX can increase blood pressure, which can increase cardiovascular risk over time. Measure blood pressure periodically. Not recommended for use in men with uncontrolled hypertension (5.4)
- Abuse of Testosterone and Monitoring of Serum Testosterone: If testosterone use at doses higher than recommended for the approved indication and in combination with other anabolic androgenic steroids is suspected, check serum testosterone concentration ( 5.5).
- Potential for Adverse Effects on Spermatogenesis: KYZATREX™ may cause azoospermia ( 5.7).
- Edema: Edema, with or without congestive heart failure (CHF), may occur in patients with pre-existing cardiac, renal, or hepatic disease. Discontinue KYZATREX™ and initiate appropriate workup ( 5.9).
- Sleep Apnea: KYZATREX™ may potentiate sleep apnea in those with risk factors ( 5.10)
- Lipid Changes: KYZATREX™ may affect serum lipid profile. Monitor patient lipid concentrations periodically; if necessary, adjust dosage of lipid lowering drug(s) or discontinue KYZATREX™ ( 5.12).
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are discussed elsewhere in the labeling:
- Polycythemia [see Warnings and Precautions (5.1)]
- Venous Thromboembolism [see Warnings and Precautions (5.2)]
- Worsening of Benign Prostatic Hyperplasia (BPH) and Potential Risk of Prostate Cancer [see Warnings and Precautions (5.3)]
- Blood Pressure Increases [see Warnings and Precautions (5.4)]
- Hepatic Adverse Effects [see Warnings and Precautions(5.8)]
- Edema [see Warnings and Precautions (5.9)]
- Sleep Apnea [see Warnings and Precautions (5.10)]
- Gynecomastia [see Warnings and Precautions (5.11)]
- Lipid Changes [see Warnings and Precautions (5.12)]
- Hypercalcemia [see Warnings and Precautions (5.13)]
- Decreased Thyroxine-binding Globulin [see Warnings and Precautions (5.14)]
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of KYZATREX™ was evaluated in Study MRS-TU-2019EXT in 155 hypogonadal males [see Clinical Studies (14)]. All patients initially received KYZATREX™ 200 mg orally twice daily. If needed, the dosage was titrated to 100 mg once daily in the morning or 100 mg, 300 mg, or 400 mg twice daily to achieve testosterone concentrations in the normal range . After the dosage titration period, patients continued their optimized dose for the remainder of the duration of the 6-month study. The mean duration of exposure was 168 days (range: 1 to 180 days). The median age was 52 years (range: 22 to 66 years); 77% were White, 19% were Black, 3% were Asian, and 2% were American Indian, Alaskan Native or Other.
Table 2 summarizes adverse reactions reported in ≥2% of patients in this 6-month study.
Table 2: Adverse Reactions in ≥ 2% of Patients Receiving KYZATREX™ in STUDY MRS-TU-2019EXT|
Adverse Reaction |
N = 155 n (%) |
|---|---|
| |
|
Hypertensión * |
4 (2.6) |
One (0.8%) patient who received KYZATREX™ experienced an adverse reaction (acne) that lead to premature discontinuation from the study.
In a 12-month, open-label study in hypogonadal adult males (N=212) who received KYZATREX™ 200 mg once daily to 400 mg twice daily (n=202) the following additional adverse reactions were reported: headache, arthralgia, diarrhea, hemoglobin increased, anxiety, constipation, peripheral edema, and PSA increased.
Blood Pressure Increases
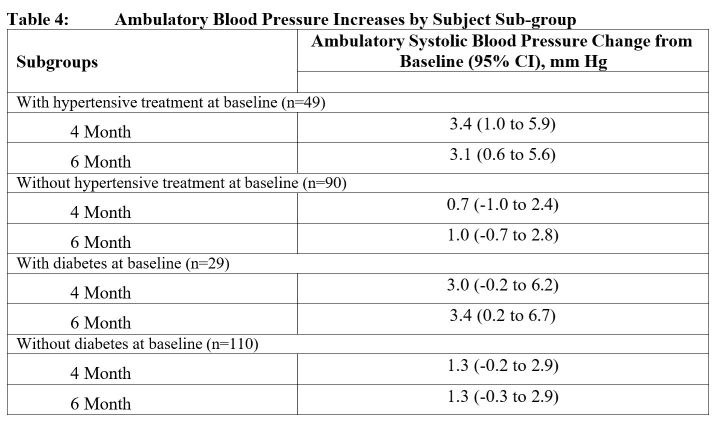
In Study MRS-TU-2019EXT, 24-hour ambulatory blood pressure monitoring (ABPM) was conducted in 155 male patients, 135 of whom completed the study. ABPM was conducted at 3 distinct 24-hour time periods: at baseline and following approximately 4 months and 6 months of treatment with KYZATREX. A total of 151 patients had acceptable 24-hour ABPM recordings at both time periods. In that group, the mean change in systolic BP from Baseline to 4 months and 6 months was + 1.7 mm Hg (95% CI 0.3, 3.1) and 1.8 mm Hg (95% CI 0.3, 3.2), respectively. In that group, the mean change in diastolic BP from Baseline to 4 months and 6 months was 0.6 mm Hg (95% CI -0.3, 1.6) and 0.6 mm Hg (95% CI -0.4, 1.6), respectively. In patients with a history of hypertension on antihypertensive therapy at baseline, the mean ABPM systolic blood pressure increased from Baseline to 4 months and 6 months by 3.4 mm Hg (95% CI 1.0, 5.9) and 3.1 mm Hg (95% CI 0.6, 5.6), respectively (n=49).
In patients with no history of hypertension at baseline, the mean systolic blood pressure from Baseline increased by 0.7 mm Hg (95% CI -1.0, 2.4) at 4 months and 1.0 mm Hg (95% CI -0.7, 2.8), at six months respectively (n =90). Ambulatory (24-hour) blood pressure Changes from Baseline for study MRS- TU-2019EXT are presented in Table 3 with 95% confidence intervals. No significant difference was observed between the 4-month and 6-month Changes from Baseline
Table 3: Blood Pressure Increases|
Blood Pressure |
Change from Baseline (95% CI) mm Hg | |
|---|---|---|
|
Systolic |
Diastolic | |
|
24-Hour Ambulatory | ||
|
4 Month |
1.7 (0.3 to 3.1) |
0.6 (-0.3 to 1.6) |
|
6 Month |
1.8 (0.3 to 3.2) |
0.6 (-0.4 to 1.6) |
A history of antihypertensive treatment and diabetes mellitus at baseline were significant factors related to ambulatory SBP increases.
Table 4 presents the Least Squares Mean estimates of Change from Baseline, with 95% CI’s, for sub-populations of subjects at study start either with or without hypertensive treatment or with or without diabetes mellitus.
A total of 5 of 155 patients (3.2%) on KYZATREX in Study MRS-TU-2019EXT began taking new antihypertensive medications after study start. No patient had a dose increase in their antihypertensive medication by the end of treatment.
Of the 155 patients in Study MRS-TU-2019EXT who used KYZATREX, 4 patients (2.6%) were reported to have an adverse reaction of hypertension.
Cardiovascular Outcomes
TRAVERSE was a randomized, double-blind, cardiovascular outcomes study to assess the cardiovascular (CV) safety of topical testosterone gel compared to placebo in 5198 hypogonadal men aged 45 to 80 years with a history of CV disease or with multiple CV risk factors. The primary outcome was the incidence of the composite endpoint of major adverse cardiovascular events (MACE), consisting of CV death, non-fatal myocardial infarction (MI), and non- fatal stroke.
The mean duration of therapy was approximately 22 months. The mean duration of follow-up was 33 months. Approximately 61% of all patients discontinued topical testosterone gel or placebo therapy.
The mean patient age (±SD) was 63.3 (7.9) years, with 2452 patients ages 65 years or more (47%); 2847 (about 55%) patients had pre-existing cardiovascular disease, whereas 2357 patients (about 45%) had an elevated cardiovascular risk at baseline, and mean BMI was 35kg/m 2. Approximately 80% of patients were White, 17% were Black, and 3% were of other races or ethnic groups. Approximately 69%, 84%, and 93% had diabetes mellitus, hyperlipidemia, and hypertension, respectively.
The mean serum testosterone concentration at baseline in patients receiving topical testosterone gel was 220.4 ng/dL (n=2596). The mean serum testosterone concentrations at 12 months, 24 months, 36 months, and 48 months in patients receiving topical testosterone gel were 440.5 ng/dL (n=1683), 420.9 ng/dl (n=1125), 428.7 ng/dL (n=731), and 365.2 ng/dL (n=220), respectively.
For patients treated with topical testosterone gel, the incidence of MACE was 7.0% (n=182 events) and for those receiving placebo, the incidence of MACE was 7.3% (n=190 events). The study demonstrated non-inferiority of topical testosterone gel versus placebo because the upper bound of 95% CI was less than the pre-specified risk margin, of 1.5 for MACE (Hazard Ratio 0.96 [95% CI: 0.78, 1.17]).
Additional Adverse Reproted in TRAVERSE
Additional adverse reactions reported in TRAVERSE at an incidence rate >2% in either treatment group and greater in topical testosterone gel versus placebo included: nonfatal arrythmias warranting intervention (5.2% vs 3.3%), atrial fibrillation (3.5% vs 2.4%), acute kidney injury (2.3% vs 1.5%) and bone fracture (3.5% vs 2.5%). For the adverse reaction of bone fracture, each event was adjudicated by clinical review
Heart Rate Increases
KYZATREX™ increased mean (95%CI) 24-hour ambulatory heart rate by an average of 0.7 (-0.5 to 1.9) beats per minute (bpm) at 4 months and 1.9 (0.6 to 3.1) bpm at 6 months in Study MRS-TU-2019EXT. Changes in heart rate were similar between patients with or without hypertension or diabetes. Changes in heart rate with treatment were most prominent in the evening, 12 to 17 hours after the morning dose.
Increases in Hemoglobin
Increases in hemoglobin were reported in 7 out of 155 patients (4.5%) in Study MRS-TU2019EXT. None of these increases led to premature discontinuation of KYZATREX™.
Hematocrit was not assessed in this study.
Headaches
Headaches were reported in 3 of 155 patients (1.9%) receiving KYZATREX™ in Study MRSTU-2019EXT.
Increases in Serum PSA
Four out of 155 patients (2.6%) receiving KYZATREX™ in Study MRS-TU-2019EXT had an increase in PSA from baseline greater than 1.4 ng/mL and two out of 155 patients (1.3%) had a PSA of at least 4.0 ng/mL during Study MRS-TU-2019EXT. The mean (SE) increase in PSA from baseline was 0.15 (±0.04) ng/mL at 6 months (n=135).
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of testosterone. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiovascular Disorders: myocardial infarction, stroke
Vascular Disorders: Venous thromboembolism
Most common adverse reactions (incidence ≥ 2%): hypertension ( 6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Marius Pharmaceuticals at 1-833-949-5040 or FDA at 1-800-FDA-1088 orwww.fda.gov/medwatch.
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Insulin
Changes in insulin sensitivity or glycemic control may occur in patients treated with androgens. In diabetic patients, the metabolic effects of androgens may decrease blood glucose and therefore necessitate a decrease in the dose of anti-diabetic medication.
7.2 Oral Vitamin K Antagonist Anticoagulants
Changes in anticoagulant activity may be seen with androgens; therefore, more frequent monitoring of international normalized ratio (INR) and prothrombin time are recommended in patients taking warfarin, especially at the initiation and termination of androgen therapy.
7.3 Corticosteroids
The concurrent use of testosterone with corticosteroids may result in increased fluid retention and requires careful monitoring particularly in patients with cardiac, renal, or hepatic disease.
7.4 Medications that May Also Increase Blood Pressure
Some prescription medications and nonprescription analgesic and cold medications contain drugs known to increase blood pressure. Concomitant administration of these medications with KYZATREX™ may lead to additional increases in blood pressure [see Warnings and Precautions (5.4)].
- Insulin: In patients with diabetes, concomitant use with KYZATREX™ may decrease blood glucose and insulin requirements ( 7.1).
- Oral Anticoagulants: Concomitant use with KYZATREX™ may cause changes in anticoagulant activity. Monitor International Normalized Ratio (INR) and prothrombin time (PT) frequently ( 7.2).
- Corticosteroids: Concomitant use with KYZATREX™ may result in increased fluid retention. Use with caution, particularly in patients with cardiac, renal, or hepatic disease ( 7.3).
- Drugs that May Also Increase Blood Pressure: Concomitant use with KYZATREX™ may lead to additional increases in blood pressure ( 7.4).
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Endogenous androgens, including testosterone and dihydrotestosterone (DHT), are responsible for the normal growth and development of the male sex organs and for maintenance of secondary sex characteristics. These effects include the growth and maturation of prostate, seminal vesicles, penis, and scrotum; the development of male hair distribution, such as facial, pubic, chest, and axillary hair; laryngeal enlargement; vocal cord thickening; alterations in body musculature; and fat distribution.
Male hypogonadism, a clinical syndrome resulting from insufficient secretion of testosterone, has two main etiologies. Primary hypogonadism is caused by defects of the gonads, such as Klinefelter syndrome or Leydig cell aplasia, whereas secondary hypogonadism (also known as hypogonadotropic hypogonadism) is the failure of the hypothalamus (or pituitary gland) to produce sufficient gonadotropins (FSH, LH).
12.2 Pharmacodynamics
There is insufficient data to characterize an exposure-response relationship or time course of pharmacodynamics.
12.3 Pharmacokinetics
Absorption
KYZATREX™ was taken orally at a starting dose of 200 mg twice per day with meals in a multicenter, open-label trial in hypogonadal males. The dose was adjusted, as needed, on Days 28 and 56 from a minimum dose of 100 mg (morning- only) to a maximum dose of 400 mg twice per day based on the plasma testosterone concentration obtained by a single blood draw collected 3 to 5 hours after the morning dose. The average daily NaF/EDTA plasma testosterone concentration was 393.3 (±113.6) ng/dL after 90 days of treatment (normal eugonadal range in NaF/EDTA plasma: 222-800 ng/dL. Note that the titration scheme for use in clinical practice is based on serum total testosterone [see Dosage and Administration (2.2)] .
KYZATREX™ is expected to produce testosterone concentrations that approximate normal concentrations seen in healthy men.
Table 5 summarizes the pharmacokinetic (PK) parameters for plasma total testosterone in patients completing at least 90 days of KYZATREX™ treatment administered daily.
Table 5: NaF-EDTA Plasma Testosterone C avg and C max at Day 90 Visit|
PK Parameter |
Plasma (N=130) | |
|---|---|---|
|
PK = pharmacokinetic; C avg = 24-hour average concentration; C max = maximum concentration | ||
|
C avg (ng/dL) |
n |
127 |
|
Mean |
393.3 | |
|
SD |
113.6 | |
|
C max (ng/dL) |
n |
130 |
|
Mean |
852.4 | |
|
SD |
311.3 |
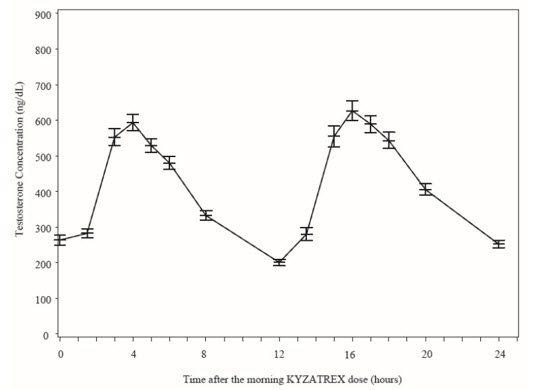
Figure 2 summarizes the mean plasma total testosterone profile at the final PK visit.
Figure 2: Mean (±SEM) Concentration-Time Profile for NaF-EDTA Plasma Total Testosterone in KYZATREX™-Treated Patients at Day 90 Visit
|
|
|
SEM = standard error of the mean |
When KYZATREX™ was given with breakfast containing 16%, 33%, and 45% fat, the exposure (AUC 0-24 hr) of testosterone was increased by 37%, 87%, and 94%, respectively, compared to when given under fasted conditions. The primary efficacy and safety study was conducted under fed conditions regardless of the type of meals and the primary efficacy endpoint of achieving testosterone C avg in normal testosterone range was met.
There was no effect on testosterone PK when KYZATREX™ was administered with 20% alcohol along with a high-fat meal versus a high-fat meal alone.
Distribution
Circulating testosterone is primarily bound in serum to sex hormone-binding globulin (SHBG) and albumin. Approximately 40% of testosterone in plasma is bound to SHBG, 2% remains unbound (free), and the rest is loosely bound to albumin and other proteins.
Metabolism
The androgenic activity of testosterone undecanoate occurs after the ester bond linking the testosterone to the undecanoic acid is cleaved by endogenous non-specific esterases.
Undecanoic acid is metabolized like all fatty acids via the beta-oxidation pathway.
Testosterone is metabolized to various 17-keto steroids through two different pathways. The major active metabolites of testosterone are dihydrotestosterone (DHT) and estradiol.
Excretion
About 90% of a dose of testosterone given intramuscularly is excreted in the urine as glucuronic and sulfuric acid conjugates of testosterone and its metabolites. About 6% of a dose is excreted in the feces, mostly in the unconjugated form. Inactivation of testosterone occurs primarily in the liver.
SPL UNCLASSIFIED SECTION
Marketed by:
Marius Pharmaceuticals LLC
2301 Suger Bush Road, Suite 510
Raleigh, NC 27612
27612Issued 07/2025
SPL MEDGUIDE SECTION
|
This Medication Guide has been approved by the U.S. Food and Drug Administration |
|
Issued: 07/2025 |
|
MEDICATION GUIDE ** KYZATREX****®****(ky-ZAH-treks)** ** (testosterone undecanoate) capsules, for oral use, CIII** |
|
What is KYZATREX™?
|
|
Do not take KYZATREX**®**if you:
|
|
Before you take KYZATREX**®****, tell your healthcare provider about all of your medical conditions, including if you:**
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Taking KYZATREX**®** with certain other medicines can affect each other. Especially, tell your healthcare provider if you take:
Know the medicines you take. Ask your healthcare provider or pharmacist for a list of these medicines, if you are not sure. Keep a list of them and show it to your healthcare provider and pharmacist when you get a new medicine. |
|
How should I take KYZATREX**®****?**
|
|
What are the possible side effects of KYZATREX**®****?** KYZATREX**®**may cause serious side effects including:
Call your healthcare provider right away if you have any of the serious side effects listed above. The most common side effect of KYZATREX®is high blood pressure. Other side effects may include headache, joint or back pain, diarrhea, increased red blood cell count, anxiety, constipation, swelling of the legs, and increased prostate specific antigen (PSA) levels. Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of KYZATREX ®. For more information, ask your healthcare provider or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
How should I store KYZATREX®?
Keep KYZATREX®and all medicines out of the reach of children. How should I throw away (dispose of) KYZATREX®?
|
|
General information about the safe and effective use of KYZATREX® Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use KYZATREX ® for a condition for which it was not prescribed. Do not give KYZATREX ® to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about KYZATREX ® that is written for health professionals. |
|
What are the ingredients in KYZATREX®? Active ingredient: testosterone undecanoate Inactive ingredients: DL-alpha-tocopheryl acetate (Vitamin E), phytosterol esters, polyoxyl 40 hydrogenated castor oil and propylene glycol monolaurate. The ingredients of the gelatin capsule shells are gelatin, glycerin, purified water, sorbitol, and titanium dioxide. Marketed by: Marius Pharmaceuticals Raleigh, NC 27615 For more information, go towww.KYZATREX.com or call 1-833-949-5040 |
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
KYZATREX™ is contraindicated in pregnant women and not indicated for use in females [see Contraindications (4)]. Testosterone is teratogenic and may cause fetal harm when administered to a pregnant woman based on data from animal studies (see Data) and its mechanism of action [see Clinical Pharmacology (12.1)]. Exposure of a female fetus to androgens may result in varying degrees of virilization. In animal developmental studies, exposure to testosterone in utero resulted in hormonal and behavioral changes in offspring and structural impairments of reproductive tissues in female and male offspring. These studies do not meet current standards for nonclinical development toxicity studies.
Data
Animal Data
In developmental studies conducted in rats, rabbits, pigs, sheep, and rhesus monkeys, pregnant animals received intramuscular injections of testosterone during the period of organogenesis. Testosterone treatment at doses that were comparable to those used for testosterone replacement therapy resulted in structural impairments in both female and male offspring. Structural impairments observed in females included increased anogenital distance, phallus development, empty scrotum, no external vagina, intrauterine growth retardation, reduced ovarian reserve, and increased ovarian follicular recruitment. Structural impairments seen in male offspring included increased testicular weight, larger seminal tubular lumen diameter, and higher frequency of occluded tubule lumen. Increased pituitary weight was seen in both sexes.
Testosterone exposure in utero also resulted in hormonal and behavioral changes in offspring. Hypertension was observed in pregnant female rats and their offspring exposed to doses approximately twice those used for testosterone replacement therapy.
8.2 Lactation
Risk Summary
KYZATREX™ is not indicated for use in females.
8.3 Females and Males of Reproductive Potential
Infertility
Males
During treatment with large doses of exogenous androgens, including KYZATREX™, spermatogenesis may be suppressed through feedback inhibition of the hypothalamicpituitary-testicular axis [see[See Warnings and Precautions (5.7) and Impairment of Fertility (13.1)] , possibly leading to adverse effects on semen parameters including sperm count. Reduced fertility has been observed in some men taking testosterone replacement therapy. Testicular atrophy, subfertility, and infertility have also been reported in men who abuse anabolic androgenic steroids [see Drug Abuse and Dependence (9.2)]. With either type of use, the impact on fertility may be irreversible.
8.4 Pediatric Use
The safety and efficacy of KYZATREX™ in pediatric patients less than 18 years old have not been established. KYZATREX™ is not recommended for use in patients less than 18 years of age because of the potential for acceleration of bone age and premature closure of epiphyses.
8.5 Geriatric Use
Clinical studies of KYZATREX™ did not include any patients 65 years of age and older. Therefore, it cannot be determined whether these patients respond differently from younger adult patients. Additionally, there are insufficient long-term safety data in geriatric patients to assess the potentially increased risk of cardiovascular disease and prostate cancer.
Geriatric patients treated with androgens including KYZATREX™ may be at risk for worsening of signs and symptoms of BPH [see (see Warnings and Precautions (5.3)].
Geriatric Patients: Geriatric patients treated with androgens may also be at risk for worsening of signs and symptoms of BPH and hypertension ( 8.5).
DESCRIPTION SECTION
11 DESCRIPTION
KYZATREX™ is provided as a gelatin capsule containing testosterone undecanoate, a fatty-acid ester of testosterone. Testosterone undecanoate is a white to off-white yellow crystalline powder. Testosterone, an androgen, is formed by cleavage of the ester side chain of testosterone undecanoate.
Testosterone undecanoate is chemically described as 17β-hydroxyandrost-4-en-3-one undecanoate. It has the empirical formula of C 30H 48O 3 and a molecular weight of 456.7 g/mol. The structural formula for testosterone undecanoate is presented in Figure 1.
Figure 1: Testosterone Undecanoate
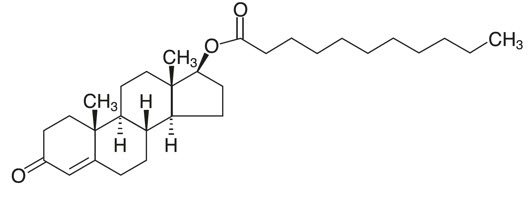
KYZATREX™ (testosterone undecanoate) capsules for oral use are available in three dosage strengths- 100 mg, 150 mg, and 200 mg. The 100 mg strength is an opaque, white capsule imprinted with "MP100" in red ink. The 150 mg strength is an opaque white capsule imprinted with "MP150" in red ink. The 200 mg strength is an opaque white capsule imprinted with "MP200" in red ink. All capsule strengths also contain DL-alpha-tocopheryl acetate (Vitamin E), phytosterol esters, polyoxyl 40 hydrogenated castor oil, and propylene glycol monolaurate as inactive ingredients.
Gelatin capsule shells are composed of the following inactive ingredients: gelatin, glycerin, purified water, sorbitol, and titanium dioxide.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
**KYZATREX™**capsules are available in three strengths of 100 mg, 150 mg, and 200 mg packaged as 60, 90 and 120 units in wide-mouth, round, white HDPE bottles with white, polypropylene, child resistant caps and induction-sealed liner
100 mg: Oval, opaque, white capsules imprinted with "MP100" in red ink supplied in bottles; NDC 80603-101-11 for 90 capsules and NDC 80603-101-22 for 120 capsules.
150 mg: Oblong, opaque, white capsules imprinted with "MP150" in red ink supplied in bottles; NDC 80603-103-11 for 90 capsules and NDC 80603-103-22 for 120 capsules.
200 mg: Oblong, opaque, white capsules imprinted with "MP200" in red ink supplied in bottles; NDC 80603-105-33 for 60 capsules , NDC 80603-105-11 for 90 capsules and NDC 80603-105-22 for 120 capsules .
Store at 20°C to 25°C (68°F to 77°F), with excursions permitted between 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]. Store the capsules in a dry place avoiding exposure to excessive moisture and humid conditions.
Dispose of unused KYZATREX™ via a take-back option. If a take-back option is unavailable, follow FDA instructions at www.fda.gov/drugdisposal.
RECENT MAJOR CHANGES SECTION
RECENT MAJOR CHANGES
Boxed Warnings, Blood Preassure Increases Removed 7/2025
Warning and Precautions, Venous Thromboembolism (5.2) 7/2025
Warnings and Precautions, Blood Preasure Increases (5.4) 7/2025
Warnings and Precautions, Cardiovascular Risk (5.4) Removed 7/2025
Boxed Warnings, Blood Preassure Increases Removed 7/2025
Warning and Precautions, Venous Thromboembolism (5.2) 7/2025
Warnings and Precautions, Blood Preasure Increases (5.4) 7/2025
Warnings and Precautions, Cardiovascular Risk (5.4) Removed 7/2025
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage Information
KYZATREX is not substitutable with other oral testosterone undecanoate products.
2.2 Confirmation of Hypogonadism Before Initiation of KYZATREX™
Prior to initiating KYZATREX™, confirm the diagnosis of hypogonadism by ensuring that serum testosterone concentrations have been measured in the morning on at least two separate days and that these testosterone concentrations are below the normal range.
2.3 Recommended Dosage and Administration
Individualize the dosage of KYZATREX™ based on the patient's serum testosterone concentration response to the drug.
The recommended starting dose is 200 mg orally twice daily, once in the morning and once in the evening. Take KYZATREX™ with food.
Dosage Adjustment
Check serum testosterone concentrations 7 days after starting treatment or after dosage adjustment, 3 to 5 hours after the morning dose. Adjust the KYZATREX™ dose as necessary as shown in Table 1. Thereafter, periodically monitor serum testosterone concentrations.
The minimum recommended dose is 100 mg once daily in the morning. The maximum recommended dose is 400 mg twice daily. For total daily doses greater than 100 mg, administer the same dose in the morning and evening.
Table 1: KYZATREX™ Dosage Adjustment Scheme|
Serum Testosterone Concentration |
Current KYZATREX™ Dosage |
New KYZATREX™ Dosage |
|---|---|---|
|
Less than 460 ng/dL |
100 mg with breakfast only |
100 mg twice daily with meals |
|
100 mg twice daily with meals |
200 mg twice daily with meals | |
|
200 mg twice daily with meals |
300 mg twice daily with meals | |
|
300 mg twice daily with meals |
400 mg twice daily with meals | |
|
460 to 971 ng/dL |
No Dosage Change | |
|
More than 971 ng/dL |
400 mg twice daily with meals |
300 mg twice daily with meals |
|
300 mg twice daily with meals |
200 mg twice daily with meals | |
|
200 mg twice daily with meals |
100 mg twice daily with meals | |
|
100 mg twice daily with meals |
100 mg with breakfast only | |
|
100 mg with breakfast only |
Discontinue treatment |
- KYZATREX™ is not substitutable with other oral testosterone undecanoate products ( 2.1).
- Prior to initiating KYZATREX™, confirm the diagnosis of hypogonadism by ensuring that serum testosterone concentrations have been measured in the morning on at least two separate days and that these concentrations are below the normal range ( 2.2).
- Take KYZATREX™ with food ( 2.3).
- Starting dosage: 200 mg orally once in the morning and once in the evening ( 2.3).
- Adjust the dosage to a minimum of 100 mg once in the morning and a maximum of 400 mg twice daily based on serum testosterone drawn 3 to 5 hours after the morning dose at least 7 days after starting treatment or following dose adjustment and periodically thereafter ( 2.3).
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Polycythemia
Advise patients that KYZATREX can cause an increase in hemoglobin/hematocrit levels that may increase the risk of thromboembolic events. Advise patients about the importance of completing laboratory testing as instructed by their health care provider while on KYZATREX [see Warnings and Precautions (5.1)].
Venous Thromboebolisn
Advise patients that KYZATREX can cause venous thromboembolism. Advise patients of the signs and symptoms of venous thromboembolism, which may include the following: lower limb pain, edema, or erythema; and dyspnea or chest pain. Advise patients to promptly report the signs and symptoms of venous thromboembolism, discontinue use of KYZATREX and seek urgent medical care.
Worsening of Benign Prostatic Hyperplasia (BPH)
Advise patients that KYZATREX™ can cause increased symptoms of BPH. Advise patients to contact their health care provider if they have any prostate- related symptoms [see Warnings and Precautions (5.3)].
Blood Pressure Increases
Inform patients that KYZATREX can increase blood pressure (BP) which can increase cardiovascular risk over time Instruct patients about the importance of monitoring BP periodically while on KYZATREX. If BP increases while on KYZATREX, antihypertensive medications may need to be started, added, or adjusted to control BP, or KYZATREX may need to be discontinued [see Warnings and Precautions (5.4)]
Edema
Advise patients that KYZATREX™ can cause edema in patients with preexisting cardiac, renal, or hepatic disease. Advise patients to notify their health care provider if edema develops or worsens [see Warnings and Precautions (5.9)].
Sleep Apnea
Advise patients that KYZATREX™ can worsen sleep apnea especially in patients with risk factors such as obesity or chronic lung diseases ™ [see Warnings and Precautions (5.10)].
Gynecomastia
Advise patients that KYZATREX™ can cause gynecomastia [see Warnings and Precautions (5.11)].
Administration Instructions
Advise patients to take KYZATREX™ with food [see Dosage and Administration (2.3)].