CLEMASZ
CLEMASZ ™ (Clemastine Fumarate Tablets, USP) Rx only
861f5615-297e-44af-a7eb-1095dc29d0a0
HUMAN PRESCRIPTION DRUG LABEL
May 3, 2025
New HeightsRx, LLC
DUNS: 119177168
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Clemastine Fumarate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 2.68 mg Tablet Bottle Label
NDC 84044-268-03
Clemastine
Fumarate
Tablets, USP
2.68 mg
Rx only
30 Tablets
New HeightsRx, LLC.
DESCRIPTION SECTION
DESCRIPTION
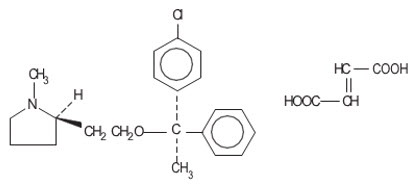
Clemastine belongs to the benzhydryl ether group of antihistaminic compounds. The chemical name is (+)-(2 R)-2-[2-[[( R)- p-Chloro-α-methyl-α- phenylbenzyl]-oxy]ethyl]-1-methylpyrrolidine fumarate (1:1).
|
| |
|
C 21H 26C1NO∙C 4H 4O 4 |
M.W. 459.97 |
Each tablet for oral administration contains 2.68 mg of clemastine fumarate, USP.
Inactive ingredients: colloidal silicon dioxide, corn starch, lactose monohydrate, povidone, pregelatinized starch and stearic acid.