Dabigatran Etexilate
These highlights do not include all the information needed to use DABIGATRAN ETEXILATE CAPSULES safely and effectively. See full prescribing information for DABIGATRAN ETEXILATE CAPSULES. DABIGATRAN ETEXILATE capsules, for oral use Initial U.S. Approval: 2010
3c24e235-218b-438e-9456-42c18ff01913
HUMAN PRESCRIPTION DRUG LABEL
Jul 30, 2025
Camber Pharmaceuticals, Inc.
DUNS: 826774775
Products 3
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Dabigatran Etexilate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (16)
Dabigatran Etexilate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (17)
Dabigatran Etexilate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (17)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Dabigatran Etexilate Capsules 75 mg Container Label

Dabigatran Etexilate Capsules 75 mg Container Carton

Dabigatran Etexilate Capsules 110 mg Blister Carton
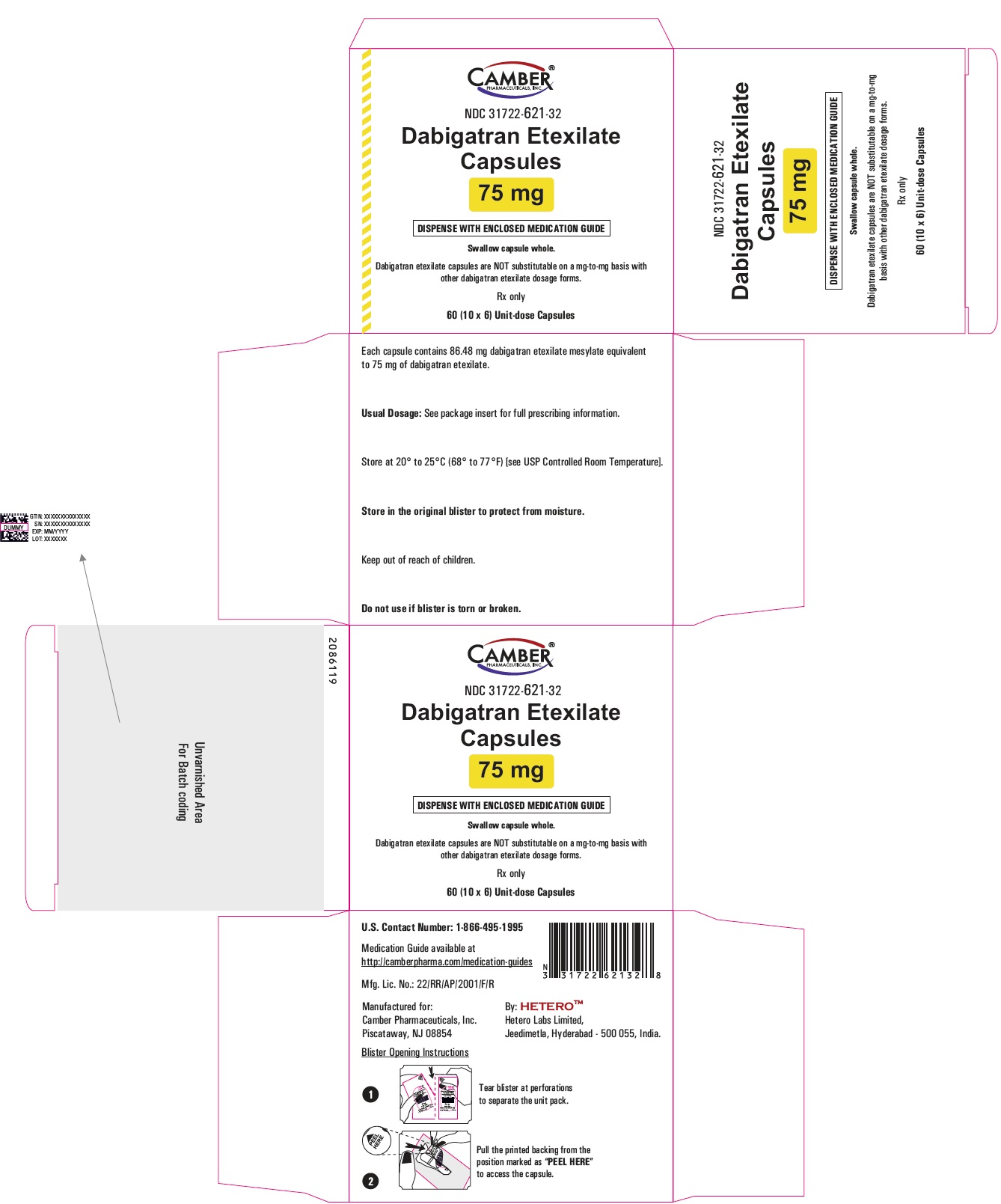
Dabigatran Etexilate Capsules 110 mg Container Label

Dabigatran Etexilate Capsules 110 mg Container Carton

Dabigatran Etexilate Capsules 110 mg Blister Carton
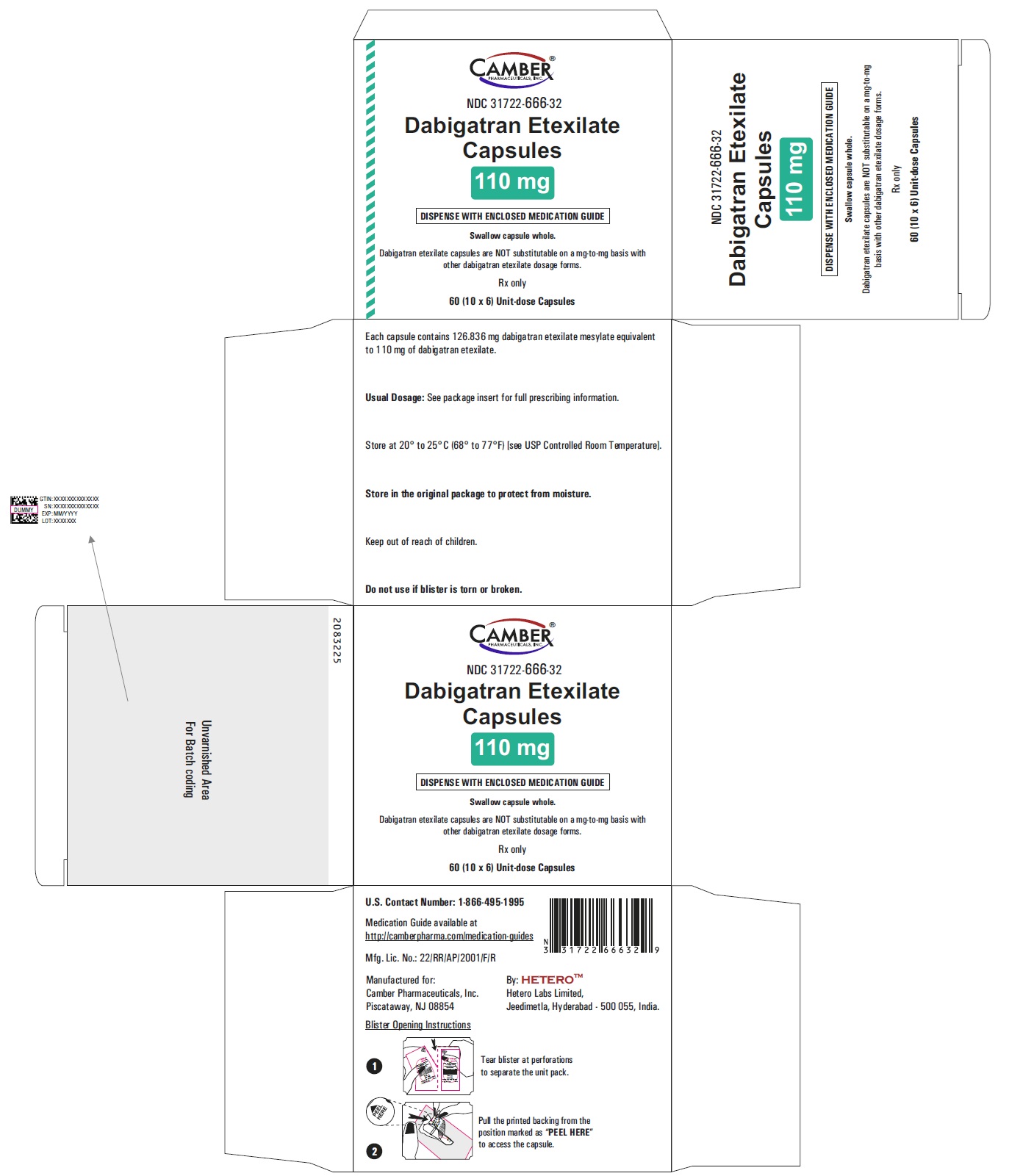
Dabigatran Etexilate Capsules 150 mg Container Label
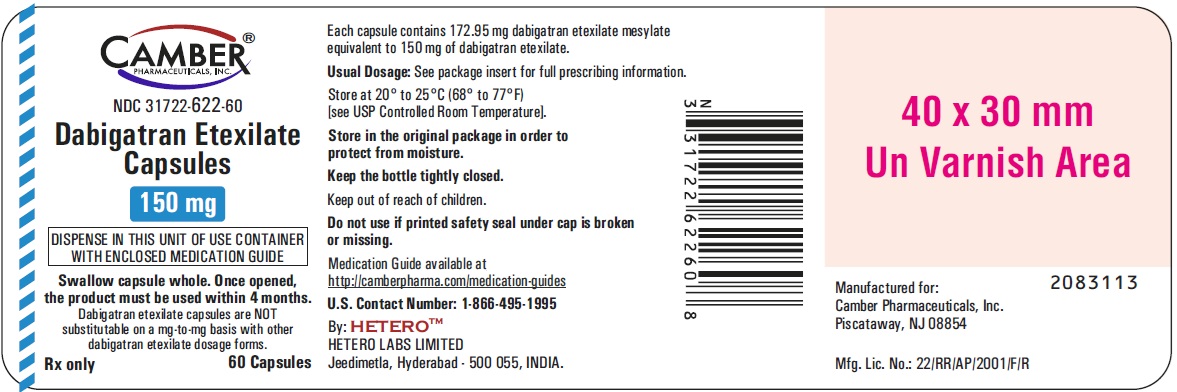
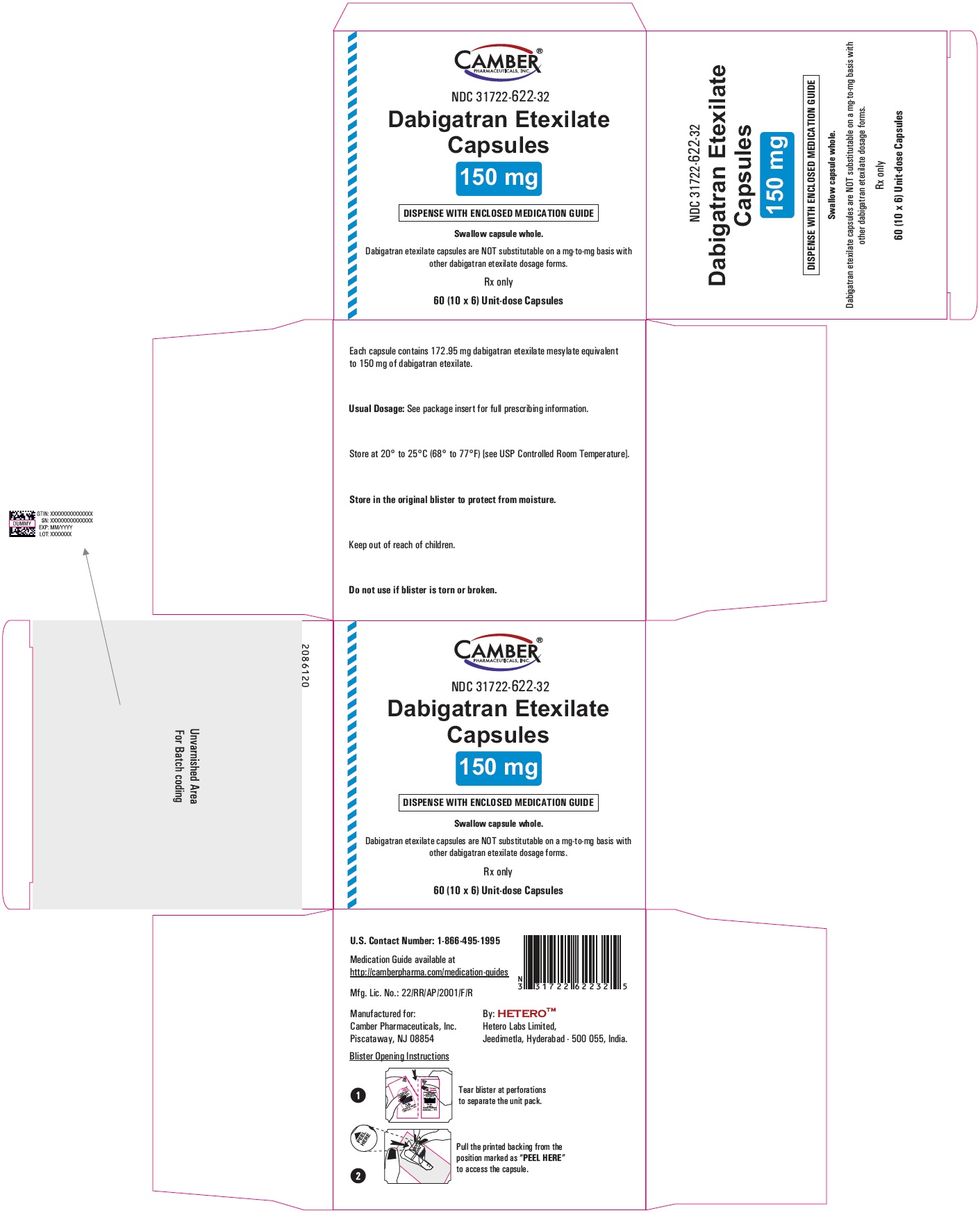
Dabigatran Etexilate Capsules 150 mg Container Carton

Dabigatran Etexilate Capsules 110 mg Blister Carton
BOXED WARNING SECTION
WARNING: (A) PREMATURE DISCONTINUATION OF DABIGATRAN ETEXILATE CAPSULES
INCREASES THE RISK OF THROMBOTIC EVENTS, and (B) SPINAL/EPIDURAL HEMATOMA
INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
1.1 Reduction of Risk of Stroke and Systemic Embolism in Non-valvular
Atrial Fibrillation in Adult Patients
Dabigatran etexilate capsules are indicated to reduce the risk of stroke and systemic embolism in adult patients with non-valvular atrial fibrillation.
1.2 Treatment of Deep Venous Thrombosis and Pulmonary Embolism in Adult
Patients
Dabigatran etexilate capsules are indicated for the treatment of deep venous thrombosis and pulmonary embolism in adult patients who have been treated with a parenteral anticoagulant for 5 to 10 days.
1.3 Reduction in the Risk of Recurrence of Deep Venous Thrombosis and
Pulmonary Embolism in Adult Patients
Dabigatran etexilate capsules are indicated to reduce the risk of recurrence of deep venous thrombosis and pulmonary embolism in adult patients who have been previously treated.
1.4 Prophylaxis of Deep Vein Thrombosis and Pulmonary Embolism in Adult
Patients Following Hip Replacement Surgery
Dabigatran etexilate capsules are indicated for the prophylaxis of deep vein thrombosis and pulmonary embolism in adult patients who have undergone hip replacement surgery.
1.5 Treatment of Venous Thromboembolic Events in Pediatric Patients
Dabigatran etexilate capsules is indicated for the treatment of venous thromboembolic events (VTE) in pediatric patients 8 to less than 18 years of age who have been treated with a parenteral anticoagulant for at least 5 days [see Dosage and Administration ( 2.3)].
1.6 Reduction in the Risk of Recurrence of Venous Thromboembolic Events in
Pediatric Patients
Dabigatran etexilate capsules is indicated to reduce the risk of recurrence of VTE in pediatric patients 8 to less than 18 years of age who have been previously treated [see Dosage and Administration ( 2.3)].
Dabigatran etexilate capsules are a direct thrombin inhibitor indicated:
• To reduce the risk of stroke and systemic embolism in adult patients with
non-valvular atrial fibrillation ( 1.1)
• For the treatment of deep venous thrombosis (DVT) and pulmonary embolism
(PE) in adult patients who have been treated with a parenteral anticoagulant
for 5 to 10 days ( 1.2)
• To reduce the risk of recurrence of DVT and PE in adult patients who have
been previously treated ( 1.3)
• For the prophylaxis of DVT and PE in adult patients who have undergone hip replacement surgery ( 1.4)
• For the treatment of venous thromboembolic events (VTE) in pediatric
patients 8 to less than 18 years of age who have been treated with a
parenteral anticoagulant for at least 5 days ( 1.5)
• To reduce the risk of recurrence of VTE in pediatric patients 8 to less than
18 years of age who have been previously treated ( 1.6)
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
Dabigatran etexilate capsules are contraindicated in patients with:
• Active pathological bleeding [see Warnings and Precautions ( 5.2) and Adverse Reactions ( 6.1)]
• History of a serious hypersensitivity reaction to dabigatran, dabigatran
etexilate, or to one of the excipients of the product (e.g., anaphylactic
reaction or anaphylactic shock) [see Adverse Reactions ( 6.1)]
• Mechanical prosthetic heart valve [see Warnings and Precautions ( 5.4)]
• Active pathological bleeding ( 4)
• History of serious hypersensitivity reaction to dabigatran etexilate
capsules ( 4)
• Mechanical prosthetic heart valve ( 4)
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Increased Risk of Thrombotic Events after Premature Discontinuation
Premature discontinuation of any oral anticoagulant, including dabigatran etexilate capsules, in the absence of adequate alternative anticoagulation increases the risk of thrombotic events. If dabigatran etexilate capsules are discontinued for a reason other than pathological bleeding or completion of a course of therapy, consider coverage with another anticoagulant and restart dabigatran etexilate capsules as soon as medically appropriate [see Dosage and Administration ( 2.6, 2.7, 2.8)].
5.2 Risk of Bleeding
Dabigatran etexilate capsules increases the risk of bleeding and can cause significant and, sometimes, fatal bleeding. Promptly evaluate any signs or symptoms of blood loss (e.g., a drop in hemoglobin and/or hematocrit or hypotension). Discontinue dabigatran etexilate capsules in patients with active pathological bleeding [see Dosage and Administration ( 2.4)].
Risk factors for bleeding include the concomitant use of other drugs that increase the risk of bleeding (e.g., anti-platelet agents, heparin, fibrinolytic therapy, and chronic use of NSAIDs). Dabigatran etexilate capsules anticoagulant activity and half-life are increased in patients with renal impairment [see Clinical Pharmacology ( 12.2)].
Reversal of Anticoagulant Effect
In adults, a specific reversal agent (idarucizumab) for dabigatran etexilate
capsules is available when reversal of the anticoagulant effect of dabigatran
is needed:
• For emergency surgery/urgent procedures
• In life-threatening or uncontrolled bleeding
In pediatric patients, the efficacy and safety of idarucizumab have not been established.
Hemodialysis can remove dabigatran; however the clinical experience supporting the use of hemodialysis as a treatment for bleeding is limited [see Overdosage ( 10)]. Prothrombin complex concentrates, or recombinant Factor VIIa may be considered but their use has not been evaluated in clinical trials. Protamine sulfate and vitamin K are not expected to affect the anticoagulant activity of dabigatran. Consider administration of platelet concentrates in cases where thrombocytopenia is present or long-acting antiplatelet drugs have been used.
5.3 Spinal/Epidural Anesthesia or Puncture
When neuraxial anesthesia (spinal/epidural anesthesia) or spinal puncture is employed, patients treated with anticoagulant agents are at risk of developing an epidural or spinal hematoma which can result in long-term or permanent paralysis [see Boxed Warning].
To reduce the potential risk of bleeding associated with the concurrent use of dabigatran etexilate and epidural or spinal anesthesia/analgesia or spinal puncture, consider the pharmacokinetic profile of dabigatran [see Clinical Pharmacology ( 12.3)]. Placement or removal of an epidural catheter or lumbar puncture is best performed when the anticoagulant effect of dabigatran is low; however, the exact timing to reach a sufficiently low anticoagulant effect in each patient is not known.
Should the physician decide to administer anticoagulation in the context of epidural or spinal anesthesia/analgesia or lumbar puncture, monitor frequently to detect any signs or symptoms of neurological impairment, such as midline back pain, sensory and motor deficits (numbness, tingling, or weakness in lower limbs), bowel and/or bladder dysfunction. Instruct patients to immediately report if they experience any of the above signs or symptoms. If signs or symptoms of spinal hematoma are suspected, initiate urgent diagnosis and treatment including consideration for spinal cord decompression even though such treatment may not prevent or reverse neurological sequelae.
5.4 Thromboembolic and Bleeding Events in Patients with Prosthetic Heart
Valves
The safety and efficacy of dabigatran etexilate capsules in adult patients
with bileaflet mechanical prosthetic heart valves was evaluated in the RE-
ALIGN trial, in which patients with bileaflet mechanical prosthetic heart
valves (recently implanted or implanted more than three months prior to
enrollment) were randomized to dose-adjusted warfarin or 150 mg, 220 mg, or
300 mg of dabigatran etexilate capsules twice a day. RE-ALIGN was terminated
early due to the occurrence of significantly more thromboembolic events (valve
thrombosis, stroke, transient ischemic attack, and myocardial infarction) and
an excess of major bleeding (predominantly post-operative pericardial
effusions requiring intervention for hemodynamic compromise) in the dabigatran
etexilate capsules treatment arm as compared to the warfarin treatment arm.
These bleeding and thromboembolic events were seen both in patients who were
initiated on dabigatran etexilate capsules postoperatively within three days
of mechanical bileaflet valve implantation, as well as in patients whose
valves had been implanted more than three months prior to enrollment.
Therefore, the use of dabigatran etexilate capsules are contraindicated in all
patients with mechanical prosthetic valves [see Contraindications ( 4)].
The use of dabigatran etexilate capsules for the prophylaxis of thromboembolic
events in patients with atrial fibrillation in the setting of other forms of
valvular heart disease, including the presence of a bioprosthetic heart valve,
has not been studied and is not recommended.
5.5 Effect of P-gp Inducers and Inhibitors on Dabigatran Exposure
The concomitant use of dabigatran etexilate capsules with P-gp inducers (e.g., rifampin) reduces exposure to dabigatran and should generally be avoided [see Clinical Pharmacology ( 12.3)].
P-gp inhibition and impaired renal function are the major independent factors that result in increased exposure to dabigatran [see Clinical Pharmacology ( 12.3)]. Concomitant use of P-gp inhibitors in patients with renal impairment is expected to produce increased exposure of dabigatran compared to that seen with either factor alone.
Reduction of Risk of Stroke and Systemic Embolism in Non-valvular Atrial
Fibrillation in Adult Patients
Reduce the dosage of dabigatran etexilate capsules to 75 mg twice daily when
dronedarone or systemic ketoconazole is co-administered with dabigatran
etexilate capsules in patients with moderate renal impairment (CrCl 30 to 50
mL/min). Avoid use of dabigatran etexilate capsules and P-gp inhibitors in
patients with severe renal impairment (CrCl 15 to 30 mL/min) [see Drug Interactions ( 7.1) and Use in Specific Populations ( 8.6)].
Treatment and Reduction in the Risk of Recurrence of Deep Venous Thrombosis
and Pulmonary Embolism in Adult Patients
Avoid use of dabigatran etexilate capsules and concomitant P-gp inhibitors in
patients with CrCl < 50 mL/min [see Drug Interactions ( 7.2) and Use in Specific Populations ( 8.6)].
Prophylaxis of Deep Vein Thrombosis and Pulmonary Embolism in Adult Patients
Following Hip Replacement Surgery
Avoid use of dabigatran etexilate capsules and concomitant P-gp inhibitors in
patients with CrCl < 50 mL/min [see Drug Interactions ( 7.3) and Use in Specific Populations ( 8.6)].
Treatment and reduction in risk of recurrence of VTE in pediatric patients
The concomitant use of dabigatran etexilate capsules with P-gp-inhibitors has not been studied in pediatric patients but may increase exposure to dabigatran.
5.6 Increased Risk of Thrombosis in Patients with Triple-Positive
Antiphospholipid Syndrome
Direct-acting oral anticoagulants (DOACs), including dabigatran etexilate capsules, are not recommended for use in patients with triple-positive antiphospholipid syndrome (APS). For patients with APS (especially those who are triple-positive [positive for lupus anticoagulant, anticardiolipin, and anti-beta 2-glycoprotein I antibodies]), treatment with DOACs has been associated with increased rates of recurrent thrombotic events compared with vitamin K antagonist therapy.
• Bleeding: Dabigatran etexilate capsules can cause serious and fatal bleeding
( 5.2)
• Bioprosthetic heart valves: Dabigatran etexilate capsules use not
recommended ( 5.4)
• Increased Risk of Thrombosis in Patients with Triple-Positive
Antiphospholipid Syndrome: Dabigatran etexilate capsules use not recommended (
5.6)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere
in the labeling:
• Increased Risk of Thrombotic Events after Premature Discontinuation [see Warnings and Precautions ( 5.1)]
• Risk of Bleeding [see Warnings and Precautions ( 5.2)]
• Spinal/Epidural Anesthesia or Puncture [see Warnings and Precautions ( 5.3)]
• Thromboembolic and Bleeding Events in Patients with Prosthetic Heart Valves
[see Warnings and Precautions ( 5.4)]
• Increased Risk of Thrombosis in Patients with Triple-Positive
Antiphospholipid Syndrome [see Warnings and Precautions ( 5.6)]
The most serious adverse reactions reported with dabigatran etexilate capsules
were related to bleeding [see Warnings and Precautions ( 5.2)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reactions rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adult Trials
Reduction of Risk of Stroke and Systemic Embolism in Non-valvular Atrial
Fibrillation
The RE-LY (Randomized Evaluation of Long-term Anticoagulant Therapy) study
provided safety information on the use of two doses of dabigatran etexilate
capsules and warfarin [see Clinical Studies ( 14.1)]. The numbers of patients
and their exposures are described in Table 2. Limited information is presented
on the 110 mg dosing arm because this dose is not approved.
Table 2 Summary of Treatment Exposure in RE-LY
|
Dabigatran Etexilate Capsules**** |
Dabigatran Etexilate Capsules**** |
Warfarin | |
|
Total number treated |
5,983 |
6,059 |
5,998 |
|
Exposure | |||
|
4,936 |
4,939 |
5,193 |
|
2,387 |
2,405 |
2,470 |
|
Mean exposure (months) |
20.5 |
20.3 |
21.3 |
|
Total patient-years |
10,242 |
10,261 |
10,659 |
Drug Discontinuation in RE-LY
The rates of adverse reactions leading to treatment discontinuation were 21%
for dabigatran etexilate capsules 150 mg and 16% for warfarin. The most
frequent adverse reactions leading to discontinuation of dabigatran etexilate
capsules were bleeding and gastrointestinal events (i.e., dyspepsia, nausea,
upper abdominal pain, gastrointestinal hemorrhage, and diarrhea).
Bleeding [see Warnings and Precautions ( 5.2)]
Table 3 shows the number of adjudicated major bleeding events during the
treatment period in the RE-LY study, with the bleeding rate per 100 subject-
years (%). Major bleeding is defined as bleeding accompanied by one or more of
the following: a decrease in hemoglobin of ≥ 2 g/dL, a transfusion of ≥ 2
units of packed red blood cells, bleeding at a critical site or with a fatal
outcome. Intracranial hemorrhage included intracerebral (hemorrhagic stroke),
subarachnoid, and subdural bleeds.
Table 3 Adjudicated Major Bleeding Events in Treated Patients****a
|
Event |
Dabigatran Etexilate Capsules |
Warfarin |
Dabigatran Etexilate Capsules |
|
Major Bleeding c |
350 (3.47) |
374 (3.58) |
0.97 (0.84, 1.12) |
|
Intracranial Hemorrhage (ICH) d |
23 (0.22) |
82 (0.77) |
0.29 (0.18, 0.46) |
|
Hemorrhagic Stroke e |
6 (0.06) |
40 (0.37) |
0.16 (0.07, 0.37) |
|
Other ICH |
17 (0.17) |
46 (0.43) |
0.38 (0.22, 0.67) |
|
Gastrointestinal |
162 (1.59) |
111 (1.05) |
1.51 (1.19, 1.92) |
|
Fatal Bleeding f |
7 (0.07) |
16 (0.15) |
0.45 (0.19, 1.10) |
|
ICH |
3 (0.03) |
9 (0.08) |
0.35 (0.09, 1.28) |
|
Non-intracranial g |
4 (0.04) |
7 (0.07) |
0.59 (0.17, 2.02) |
aPatients during treatment or within 2 days of stopping study treatment. Major
bleeding events within each subcategory were counted once per patient, but
patients may have contributed events to multiple subcategories.
bAnnual event rate per 100 pt-years = 100 * number of subjects with
event/subject-years. Subject-years is defined as cumulative number of days
from first drug intake to event date, date of last drug intake + 2, death date
(whatever occurred first) across all treated subjects divided by 365.25. In
case of recurrent events of the same category, the first event was considered.
cDefined as bleeding accompanied by one or more of the following: a decrease
in hemoglobin of ≥ 2 g/dL, a transfusion of 2 or more units of packed red
blood cells, bleeding at a critical site or with fatal outcome.
dIntracranial bleed included intracerebral (hemorrhagic stroke), subarachnoid,
and subdural bleeds.
eOn-treatment analysis based on the safety population, compared to ITT
analysis presented in Section 14 Clinical Studies.
fFatal bleed: Adjudicated major bleed as defined above with investigator
reported fatal outcome and adjudicated death with primary cause from bleeding.
gNon-intracranial fatal bleed: Adjudicated major bleed as defined above and
adjudicated death with primary cause from bleeding but without symptomatic
intracranial bleed based on investigator’s clinical assessment.
There was a higher rate of any gastrointestinal bleeds in patients receiving
dabigatran etexilate capsules 150 mg than in patients receiving warfarin (6.6%
vs 4.2%, respectively).
The risk of major bleeds was similar with dabigatran etexilate capsules 150 mg
and warfarin across major subgroups defined by baseline characteristics (see
Figure 1), with the exception of age, where there was a trend toward a higher
incidence of major bleeding on dabigatran etexilate capsules (hazard ratio
1.2, 95% CI: 1.0 to 1.5) for patients ≥ 75 years of age.
Figure 1 Adjudicated Major Bleeding by Baseline Characteristics Including Hemorrhagic Stroke Treated Patients
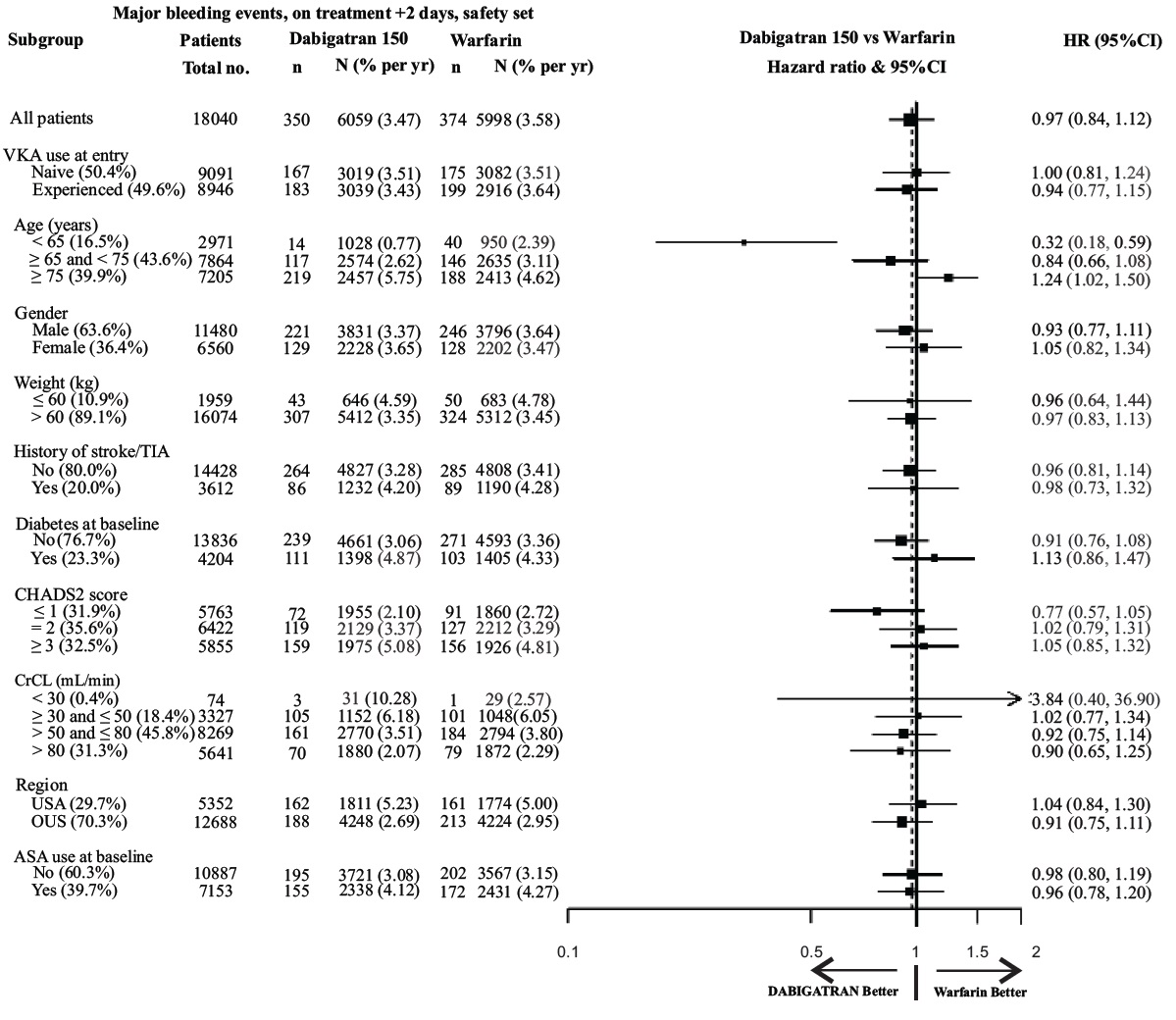
Note: The figure above presents effects in various subgroups all of which are baseline characteristics and all of which were pre-specified. The 95% confidence limits that are shown do not take into account how many comparisons were made, nor do they reflect the effect of a particular factor after adjustment for all other factors. Apparent homogeneity or heterogeneity among groups should not be over-interpreted.
Gastrointestinal Adverse Reactions
Patients on dabigatran etexilate capsules 150 mg had an increased incidence of
gastrointestinal adverse reactions (35% vs 24% on warfarin). These were
commonly dyspepsia (including abdominal pain upper, abdominal pain, abdominal
discomfort, and epigastric discomfort) and gastritis-like symptoms (including
GERD, esophagitis, erosive gastritis, gastric hemorrhage, hemorrhagic
gastritis, hemorrhagic erosive gastritis, and gastrointestinal ulcer).
Hypersensitivity Reactions
In the RE-LY study, drug hypersensitivity (including urticaria, rash, and
pruritus), allergic edema, anaphylactic reaction, and anaphylactic shock were
reported in < 0.1% of patients receiving dabigatran etexilate capsules.
Treatment and Reduction in the Risk of Recurrence of Deep Venous Thrombosis
and Pulmonary Embolism
Dabigatran etexilate capsules were studied in 4,387 patients in 4 pivotal,
parallel, randomized, double-blind trials. Three of these trials were active-
controlled (warfarin) (RE-COVER, RE-COVER II, and RE-MEDY), and one study (RE-
SONATE) was placebo-controlled. The demographic characteristics were similar
among the 4 pivotal studies and between the treatment groups within these
studies. Approximately 60% of the treated patients were male, with a mean age
of 55.1 years. The majority of the patients were white (87.7%), 10.3% were
Asian, and 1.9% were black with a mean CrCl of 105.6 mL/min.
Bleeding events for the 4 pivotal studies were classified as major bleeding events if at least one of the following criteria applied: fatal bleeding, symptomatic bleeding in a critical area or organ (intraocular, intracranial, intraspinal or intramuscular with compartment syndrome, retroperitoneal bleeding, intra-articular bleeding, or pericardial bleeding), bleeding causing a fall in hemoglobin level of 2.0 g/dL (1.24 mmol/L or more, or leading to transfusion of 2 or more units of whole blood or red cells).
RE-COVER and RE-COVER II studies compared dabigatran etexilate capsules 150 mg twice daily and warfarin for the treatment of deep vein thrombosis and pulmonary embolism. Patients received 5 to 10 days of an approved parenteral anticoagulant therapy followed by 6 months, with mean exposure of 164 days, of oral only treatment; warfarin was overlapped with parenteral therapy. Table 4 shows the number of patients experiencing bleeding events in the pooled analysis of RE-COVER and RE-COVER II studies during the full treatment including parenteral and oral only treatment periods after randomization.
Table 4 Bleeding Events in RE-COVER and RE-COVER II Treated Patients
|
Bleeding Events-Full Treatment Period Including Parenteral Treatment | |||
|
Dabigatran Etexilate Capsules |
Warfarin |
Hazard****Ratio | |
|
Patients |
N=2,553 |
N=2,554 | |
|
Major bleeding event a |
37 (1.4) |
51 (2.0) |
0.73 (0.48, 1.11) |
|
Fatal bleeding |
1 (0.04) |
2 (0.1) | |
|
Bleeding in a critical area or organ |
7 (0.3) |
15 (0.6) | |
|
Fall in hemoglobin ≥ 2 g/dL or transfusion ≥ 2 units of whole blood or packed red blood cells |
32 (1.3) |
38 (1.5) | |
|
Bleeding sites for MBE b | |||
|
Intracranial |
2 (0.1) |
5 (0.2) | |
|
Retroperitoneal |
2 (0.1) |
1 (0.04) | |
|
Intraarticular |
2 (0.1) |
4 (0.2) | |
|
Intramuscular |
2 (0.1) |
6 (0.2) | |
|
Gastrointestinal |
15 (0.6) |
14 (0.5) | |
|
Urogenital |
7 (0.3) |
14 (0.5) | |
|
Other |
8 (0.3) |
8 (0.3) | |
|
Clinically relevant non-major bleeding |
101 (4.0) |
170 (6.7) |
0.58 (0.46, 0.75) |
|
Any bleeding |
411 (16.1) |
567 (22.7) |
0.70 (0.61, 0.79) |
Note: MBE can belong to more than one criterion.
aPatients with at least one MBE.
bBleeding site based on investigator assessment. Patients can have more than
one site of bleeding.
cConfidence interval
The rate of any gastrointestinal bleeds in patients receiving dabigatran etexilate capsules 150 mg in the full treatment period was 3.1% (2.4% on warfarin).
The RE-MEDY and RE-SONATE studies provided safety information on the use of dabigatran etexilate capsules for the reduction in the risk of recurrence of deep vein thrombosis and pulmonary embolism.
RE-MEDY was an active-controlled study (warfarin) in which 1,430 patients received dabigatran etexilate capsules 150 mg twice daily following 3 to 12 months of oral anticoagulant regimen. Patients in the treatment studies who rolled over into the RE-MEDY study had a combined treatment duration of up to more than 3 years, with mean exposure of 473 days. Table 5 shows the number of patients experiencing bleeding events in the study.
Table 5 Bleeding Events in RE-MEDY Treated Patients
|
Dabigatran Etexilate Capsules |
Warfarin |
Hazard****Ratio | |
|
Patients |
N=1,430 |
N=1,426 | |
|
Major bleeding event a |
13 (0.9) |
25 (1.8) |
0.54 (0.25, 1.16) |
|
Fatal bleeding |
0 |
1 (0.1) | |
|
Bleeding in a critical area or organ |
7 (0.5) |
11 (0.8) | |
|
Fall in hemoglobin ≥ 2 g/dL or transfusion ≥ 2 units of whole blood or packed red blood cells |
7 (0.5) |
16 (1.1) | |
|
Bleeding sites for MBE b | |||
|
Intracranial |
2 (0.1) |
4 (0.3) | |
|
Intraocular |
4 (0.3) |
2 (0.1) | |
|
Retroperitoneal |
0 |
1 (0.1) | |
|
Intraarticular |
0 |
2 (0.1) | |
|
Intramuscular |
0 |
4 (0.3) | |
|
Gastrointestinal |
4 (0.3) |
8 (0.6) | |
|
Urogenital |
1 (0.1) |
1 (0.1) | |
|
Other |
2 (0.1) |
4 (0.3) | |
|
Clinically relevant non-major bleeding |
71 (5.0) |
125 (8.8) |
0.56 (0.42, 0.75) |
|
Any bleeding |
278 (19.4) |
373 (26.2) |
0.71 (0.61, 0.83) |
Note: MBE can belong to more than one criterion.
aPatients with at least one MBE.
bBleeding site based on investigator assessment. Patients can have more than
one site of bleeding.
cConfidence interval
In the RE-MEDY study, the rate of any gastrointestinal bleeds in patients
receiving dabigatran etexilate capsules 150 mg was 3.1% (2.2% on warfarin).
RE-SONATE was a placebo-controlled study in which 684 patients received
dabigatran etexilate capsules 150 mg twice daily following 6 to 18 months of
oral anticoagulant regimen. Patients in the treatment studies who rolled over
into the RE-SONATE study had combined treatment duration up to 9 months, with
mean exposure of 165 days. Table 6 shows the number of patients experiencing
bleeding events in the study.
Table 6 Bleeding Events in RE-SONATE Treated Patients
|
Dabigatran Etexilate Capsules |
Placebo |
Hazard****Ratio | |
|
Patients |
N=684 |
N=659 | |
|
Major bleeding event a |
2 (0.3) |
0 | |
|
Bleeding in a critical area or organ |
0 |
0 | |
|
Gastrointestinal b |
2 (0.3) |
0 | |
|
Clinically relevant non-major bleeding |
34 (5.0) |
13 (2.0) |
2.54 (1.34, 4.82) |
|
Any bleeding |
72 (10.5) |
40 (6.1) |
1.77 (1.20, 2.61) |
Note: MBE can belong to more than one criterion.
aPatients with at least one MBE.
bBleeding site based on investigator assessment. Patients can have more than
one site of bleeding.
cConfidence interval
In the RE-SONATE study, the rate of any gastrointestinal bleeds in patients receiving dabigatran etexilate capsules 150 mg was 0.7% (0.3% on placebo).
Clinical Myocardial Infarction Events
In the active-controlled VTE studies, a higher rate of clinical myocardial
infarction was reported in patients who received dabigatran etexilate capsules
[20 (0.66 per 100 patient-years)] than in those who received warfarin [5 (0.17 per 100 patient-years)]. In the placebo-controlled study, a similar rate of
nonfatal and fatal clinical myocardial infarction was reported in patients who
received dabigatran etexilate capsules [1 (0.32 per 100 patient-years)] and in
those who received placebo [1 (0.34 per 100 patient-years)].
Gastrointestinal Adverse Reactions
In the four pivotal studies, patients on dabigatran etexilate capsules 150 mg
had a similar incidence of gastrointestinal adverse reactions (24.7% vs 22.7%
on warfarin). Dyspepsia (including abdominal pain upper, abdominal pain,
abdominal discomfort, and epigastric discomfort) occurred in patients on
dabigatran etexilate capsules 7.5% vs 5.5% on warfarin, and gastritis-like
symptoms (including gastritis, GERD, esophagitis, erosive gastritis and
gastric hemorrhage) occurred at 3.0% vs 1.7%, respectively.
Hypersensitivity Reactions
In the 4 pivotal studies, drug hypersensitivity (including urticaria, rash,
and pruritus), allergic edema, anaphylactic reaction, and anaphylactic shock
were reported in 0.1% of patients receiving dabigatran etexilate capsules.
Prophylaxis of Deep Vein Thrombosis and Pulmonary Embolism Following Hip
Replacement Surgery
Dabigatran etexilate capsules were studied in 5,476 patients, randomized and
treated in two double-blind, active-controlled non-inferiority trials (RE-
NOVATE and RE-NOVATE II). The demographic characteristics were similar across
the two studies and between the treatment groups within these studies.
Approximately 45.3% of the treated patients were male, with a mean age of 63.2
years. The majority of the patients were white (96.1%), 3.6% were Asian, and
0.3% were black with a mean CrCl of 92 mL/min.
Bleeding events for the RE-NOVATE and RE-NOVATE II studies were classified as major bleeding events if at least one of the following criteria applied: fatal bleeding, symptomatic bleeding in a critical area or organ (intraocular, intracranial, intraspinal or retroperitoneal bleeding), bleeding causing a fall in hemoglobin level of 2.0 g/dL (1.24 mmol/L) or more, or leading to transfusion of 2 or more units of whole blood or red cells, requiring treatment cessation or leading to re-operation.
The RE-NOVATE study compared dabigatran etexilate capsules 75 mg taken orally 1 to 4 hours after surgery followed by 150 mg once daily, dabigatran etexilate capsules 110 mg taken orally 1 to 4 hours after surgery followed by 220 mg once daily and subcutaneous enoxaparin 40 mg once daily initiated the evening before surgery for the prophylaxis of deep vein thrombosis and pulmonary embolism in patients who had undergone hip replacement surgery. The RE-NOVATE II study compared dabigatran etexilate capsules 110 mg taken orally 1 to 4 hours after surgery followed by 220 mg once daily and subcutaneous enoxaparin 40 mg once daily initiated the evening before surgery for the prophylaxis of deep vein thrombosis and pulmonary embolism in patients who had undergone hip replacement surgery. In the RE-NOVATE and RE-NOVATE II studies, patients received 28 to 35 days of dabigatran etexilate capsules or enoxaparin with median exposure of 33 days. Tables 7 and 8 show the number of patients experiencing bleeding events in the analysis of RE-NOVATE and RE-NOVATE II.
Table 7 Bleeding Events in RE-NOVATE Treated Patients
|
Dabigatran Etexilate Capsules |
Enoxaparin | |
|
Patients |
N=1,146 |
N=1,154 |
|
Major bleeding event |
23 (2.0) |
18 (1.6) |
|
Clinically relevant non-major bleeding |
48 (4.2) |
40 (3.5) |
|
Any bleeding |
141 (12.3) |
132 (11.4) |
Table 8 Bleeding Events in RE-NOVATE II Treated Patients
|
Dabigatran Etexilate Capsules |
Enoxaparin | |
|
Patients |
N=1,010 |
N=1,003 |
|
Major bleeding event |
14 (1.4) |
9 (0.9) |
|
Clinically relevant non-major bleeding |
26 (2.6) |
20 (2.0) |
|
Any bleeding |
98 (9.7) |
83 (8.3) |
In the two studies, the rate of major gastrointestinal bleeds in patients receiving dabigatran etexilate capsules and enoxaparin was the same (0.1%) and for any gastrointestinal bleeds was 1.4% for dabigatran etexilate capsules 220 mg and 0.9% for enoxaparin.
Gastrointestinal Adverse Reactions
In the two studies, the incidence of gastrointestinal adverse reactions for
patients on dabigatran etexilate capsules 220 mg and enoxaparin was 39.5% and
39.5%, respectively. Dyspepsia (including abdominal pain upper, abdominal
pain, abdominal discomfort, and epigastric discomfort) occurred in patients on
dabigatran etexilate capsules 220 mg in 4.1% vs. 3.8% on enoxaparin, and
gastritis-like symptoms (including gastritis, GERD, esophagitis, erosive
gastritis and gastric hemorrhage) occurred at 0.6% vs 1.0%, respectively.
Hypersensitivity Reactions
In the two studies, drug hypersensitivity (such as urticaria, rash, and
pruritus) was reported in 0.3% of patients receiving dabigatran etexilate
capsules 220 mg.
Clinical Myocardial Infarction Events
In the two studies, clinical myocardial infarction was reported in 2 (0.1%) of
patients who received dabigatran etexilate capsules 220 mg and 6 (0.3%) of
patients who received enoxaparin.
Pediatric Trials
Treatment of VTE in Pediatric Patients
The safety of dabigatran etexilate A in the treatment of VTE in pediatric patients was studied in one phase III trial (DIVERSITY). The DIVERSITY study was a randomized, open-label, active-controlled, parallel-group trial comparing dabigatran etexilate with standard of care – SOC (vitamin K antagonists, low molecular weight heparin, or fondaparinux). There were 266 pediatric patients who received study treatment, 176 patients treated with dabigatran etexilate and 90 patients treated with SOC. Patients on dabigatran etexilate received age-and weight-adjusted dosages of an age-appropriate formulation of dabigatran etexilate (capsules, pellets, or oral solution) twice daily.
Patients had a median age of 14 years (range: 0 to 17 years), 92% were white, and half the patients were male (50%). Following at least 5 days of parenteral anticoagulant therapy, the median duration of treatment with dabigatran etexilate was 85 days (range: 1 to 105). Patients with estimated glomerular filtration rate (eGFR) <50 mL/min/1.73 m 2were excluded from the trial.
Bleeding
Data on adjudicated major bleeding, clinically relevant non-major (CRNM) bleeding and minor bleeding events, for the dabigatran etexilate group and the SOC group in the DIVERSITY study, are reported in Table 9. There was no statistically significant difference in the time to first major bleeding event.
Table 9 Summary of All Adjudicated Bleeding Events During On-Treatment Period in DIVERSITY
|
DABIGATRAN ETEXILATE**** |
Standard of Care (SOC) | |
|
Patients |
N=176 |
N=90 |
|
Major bleeding event 1 |
4 (2.3) |
2 (2.2) |
|
Fatal bleeding |
0 |
1 (1.1) |
|
Clinically relevant non-major bleeding |
2 (1.1) |
1 (1.1) |
|
Minor bleeding |
33 (19) |
21 (23) |
|
Major and clinically relevant non-major bleeding |
6 (3.4) |
3 (3.3) |
|
Any bleeding |
38 (22) |
22 (24) |
1Major bleeding event if at least one of the following criteria applied: fatal bleeding, symptomatic bleeding in a critical area or organ (intraocular, intracranial, intraspinal or intramuscular with compartment syndrome, retroperitoneal bleeding, intra-articular bleeding, or pericardial bleeding), bleeding causing a fall in hemoglobin level of 2.0 g/dL (1.24 mmol/L) or more, or leading to transfusion of 2 or more units of whole blood or red cells.
Site-specific bleeding rates were comparable between the two arms, with the exception of the rate of any gastrointestinal bleeds (5.7% in dabigatran etexilate arm vs 1.8% in SOC arm).
Gastrointestinal Adverse Reactions
The incidence of gastrointestinal adverse reactions for patients on dabigatran etexilate and SOC was 32% and 12%, respectively, with the following occurring in ≥ 5% of patients taking dabigatran etexilate: dyspepsia (including term gastro-esophageal reflux disease, gastric pH decreased and esophagitis) in 9% (vs 2%), upper abdominal pain in 5% (vs 1%), vomiting in 8% (vs 2%), nausea 5% (vs 4%), and diarrhea 5% (vs 1%).
Reduction in Risk of Recurrence of VTE in Pediatric Patients
The safety of dabigatran etexilate in the reduction in the risk of recurrence of VTE in pediatric patients was studied in one open-label single-arm trial (Study 2). Study 2 enrolled patients who required further anticoagulation due to the presence of a clinical risk factor after completing the initial treatment for confirmed VTE (for at least 3 months) or after completing the DIVERSITY study and received dabigatran etexilate until the clinical risk factor resolved, or up to a maximum of 12 months. There were 213 pediatric patients treated with dabigatran etexilate, in a similar fashion as in the DIVERSITY trial.
Patients had a median age of 14 years (range: 0 to 18 years), 91% were white, and 55% of patients were male. Patients previously enrolled on DIVERSITY accounted for 43% of patients enrolled on Study 2 (29% from dabigatran etexilate arm and 14% from SOC arm). The median duration of treatment with dabigatran etexilate in Study 2 was 42 weeks (range: 0 to 56 weeks), with 45% of patients completing the 12-month planned duration, 17% stopping due to resolution of VTE risk factors, 12% stopping due to failure to attain target dabigatran concentration and 6% had an adverse event leading to discontinuation.
During the on-treatment period of Study 2, 3 patients (1.4%) had a major bleeding event, 3 patients (1.4%) had a clinically relevant non-major bleeding event, and 44 patients (20%) had a minor bleeding event. The most common drug- related adverse reactions were dyspepsia (5%), epistaxis (3.3%), nausea (3.3%) and menorrhagia (2.8%).
The adverse reaction profile in pediatric patients was generally consistent with that of adult patients.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of dabigatran etexilate capsules. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and Lymphatic System Disorders:Agranulocytosis, neutropenia,
thrombocytopenia
Gastrointestinal Disorders:Esophageal ulcer
Immune System Disorders:Angioedema
Renal and Urinary Disorders:Anticoagulant-related nephropathy
Skin and Subcutaneous Tissue Disorders:Alopecia
Most common adverse reactions (> 15%) are gastrointestinal adverse reactions
and bleeding ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Hetero Labs Limited at
1-866-495-1995 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Reduction of Risk of Stroke and Systemic Embolism in Non-valvular
Atrial Fibrillation in Adult Patients
The concomitant use of dabigatran etexilate capsules with P-gp inducers (e.g., rifampin) reduces exposure to dabigatran and should generally be avoided [see Clinical Pharmacology ( 12.3)].
P-gp inhibition and impaired renal function are the major independent factors that result in increased exposure to dabigatran [see Clinical Pharmacology ( 12.3)]. Concomitant use of P-gp inhibitors in patients with renal impairment is expected to produce increased exposure of dabigatran compared to that seen with either factor alone.
In patients with moderate renal impairment (CrCl 30 to 50 mL/min), reduce the dosage of dabigatran etexilate capsules to 75 mg twice daily when administered concomitantly with the P-gp inhibitors dronedarone or systemic ketoconazole. The use of the P-gp inhibitors verapamil, amiodarone, quinidine, clarithromycin, and ticagrelor does not require a dosage adjustment of dabigatran etexilate capsules. These results should not be extrapolated to other P-gp inhibitors [see Warnings and Precautions ( 5.5), Use in Specific Populations ( 8.6), and Clinical Pharmacology ( 12.3)].
The concomitant use of dabigatran etexilate capsules and P-gp inhibitors in patients with severe renal impairment (CrCl 15 to 30 mL/min) should be avoided [see Warnings and Precautions ( 5.5), Use in Specific Populations ( 8.6), and Clinical Pharmacology ( 12.3)].
7.2 Treatment and Reduction in the Risk of Recurrence of Deep Venous
Thrombosis and Pulmonary Embolism in Adult Patients
Avoid use of dabigatran etexilate capsules and P-gp inhibitors in patients with CrCl < 50 mL/min [see Warnings and Precautions ( 5.5), Use in Specific Populations ( 8.6), and Clinical Pharmacology ( 12.3)].
7.3 Prophylaxis of Deep Vein Thrombosis and Pulmonary Embolism in Adult
Patients Following Hip Replacement Surgery
In patients with CrCl ≥ 50 mL/min who have concomitant administration of P-gp inhibitors such as dronedarone or systemic ketoconazole, it may be helpful to separate the timing of administration of dabigatran etexilate capsules and the P-gp inhibitor by several hours. The concomitant use of dabigatran etexilate capsules and P-gp inhibitors in patients with CrCl < 50 mL/min should be avoided [see Warnings and Precautions ( 5.5), Use in Specific Populations ( 8.6), and Clinical Pharmacology ( 12.2, 12.3)].
7.4 Treatment and Reduction in Risk of Recurrence of VTE in Pediatric
Patients
The concomitant use of dabigatran etexilate with P-gp inhibitors has not been studied in pediatric patients but may increase exposure to dabigatran [see Warnings and Precautions ( 5.5)].
• P-gp inducers: Avoid coadministration with dabigatran etexilate capsules (
5.5)
• P-gp inhibitors in adult patients with CrCl 30 to 50 mL/min: Reduce dosage
or avoid ( 7)
• P-gp inhibitors in adult patients with CrCl < 30 mL/min: Not recommended (
7)
DESCRIPTION SECTION
11 DESCRIPTION
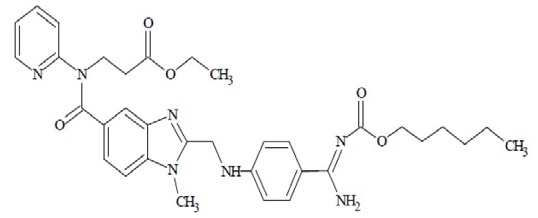
The chemical name for dabigatran etexilate mesylate, a direct thrombin inhibitor, is N-[[2-[[[4-[(hexyloxy)carbonyl]amino]iminomethyl]phenyl]amino]methyl]-1-methyl-1H-benzimidazol-5-yl]carbonyl]-N-2-pyridinyl- β-alanine ethyl ester, methanesulfonate. The empirical formula is C 34H 41N 7O 5.CH 3SO 3H and the molecular weight is 723.86 (mesylate salt), 627.75 (free base). The structural formula is:
Dabigatran etexilate mesylate is a yellow-white to yellow color powder. Sparingly soluble in methanol.
Dabigatran etexilate capsules are supplied in 75 mg, 110 mg and 150 mg strengths for oral administration. Each capsule contains dabigatran etexilate mesylate as the active ingredient: 150 mg dabigatran etexilate (equivalent to 172.95 mg dabigatran etexilate mesylate), 110 mg dabigatran etexilate (equivalent to 126.83 mg dabigatran etexilate mesylate), or 75 mg dabigatran etexilate (equivalent to 86.48 mg dabigatran etexilate mesylate) along with the following inactive ingredients: hydroxypropyl cellulose, hypromellose, sugar spheres (sucrose and corn starch), talc and tartaric acid. The capsule shell is composed of iron oxide red, iron oxide yellow, hypromellose and titanium dioxide. The capsules are printed with black ink containing black iron oxide, potassium hydroxide, propylene glycol, shellac and strong ammonia solution.
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage Information
Dabigatran etexilate is available in different dosage forms and not all dosage forms are approved for the same indications and age groups. In addition, there are differences between the dosage forms with respect to dosing due to differences in bioavailability. Do not substitute different dosage forms on a milligram-to-milligram basis and do not combine more than one dosage form to achieve the total dose [see Clinical Pharmacology ( 12.3)].
2.2 Recommended Dabigatran Etexilate Capsules Dosage for Adults
|
Indication |
Dosage | |
|
Reductionin****Riskof****Stroke****and** ****Systemic******Embolism in Non-valvular AF |
CrCl > 30 mL/min: |
150 mg twice daily |
|
CrCl 30 to 50 mL/min with |
Reduce dosage to 75 mg twice daily if given with P-gp inhibitors dronedarone or systemic ketoconazole. | |
|
CrCl < 30 mL/min with concomitant use of P-gp inhibitors: |
Avoid coadministration | |
|
Treatment****of DVT and PE Reduction in the Risk of Recurrence of DVT and PE |
CrCl > 30 mL/min: CrCl ≤ 30 mL/min or on dialysis: |
150 mg twice daily Dosing recommendations cannot be provided |
|
CrCl < 50 mL/min with concomitant use of P-gp inhibitors: |
Avoid coadministration | |
|
Prophylaxis of DVT and PE Following Hip Replacement Surgery |
CrCl > 30 mL/min: CrCl ≤ 30 mL/min or on dialysis: |
110 mg for first day, then 220 mg once daily Dosing recommendations cannot be provided |
|
CrCl < 50 mL/min with concomitant use of P-gp inhibitors: |
Avoid coadministration |
Reduction of Risk of Stroke and Systemic Embolism in Non-valvular Atrial
Fibrillation in Adult Patients
For patients with creatinine clearance (CrCl) > 30 mL/min, the recommended
dosage of dabigatran etexilate capsules is 150 mg taken orally, twice daily.
For patients with severe renal impairment (CrCl 15 to 30 mL/min), the
recommended dosage of dabigatran etexilate capsules is 75 mg twice daily [see Use in Specific Populations ( 8.6) and Clinical Pharmacology ( 12.3)]. Dosing
recommendations for patients with a CrCl < 15 mL/min or on dialysis cannot be
provided.
Treatment of Deep Venous Thrombosis and Pulmonary Embolism in Adult Patients
For patients with CrCl > 30 mL/min, the recommended dosage of dabigatran
etexilate capsules is 150 mg taken orally, twice daily, after 5 to 10 days of
parenteral anticoagulation. Dosing recommendations for patients with a CrCl ≤
30 mL/min or on dialysis cannot be provided [see Use in Specific Populations ( 8.6) and Clinical Pharmacology ( 12.3)].
Reduction in the Risk of Recurrence of Deep Venous Thrombosis and Pulmonary
Embolism in Adult Patients
For patients with CrCl > 30 mL/min, the recommended dosage of dabigatran
etexilate capsules is 150 mg taken orally, twice daily after previous
treatment. Dosing recommendations for patients with a CrCl ≤ 30 mL/min or on
dialysis cannot be provided [see Use in Specific Populations ( 8.6) and Clinical Pharmacology ( 12.3)].
Prophylaxis of Deep Vein Thrombosis and Pulmonary Embolism in Adult Patients
Following Hip Replacement Surgery
For patients with CrCl > 30 mL/min, the recommended dosage of dabigatran
etexilate capsules is 110 mg taken orally 1-4 hours after surgery and after
hemostasis has been achieved, then 220 mg taken once daily for 28-35 days. If
dabigatran etexilate capsules is not started on the day of surgery, after
hemostasis has been achieved initiate treatment with 220 mg once daily. Dosing
recommendations for patients with a CrCl ≤ 30 mL/min or on dialysis cannot be
provided [see Dosage and Administration ( 2.4), Use in Specific Populations ( 8.6), and Clinical Pharmacology ( 12.2, 12.3)].
2.3 Recommended Dabigatran Etexilate Capsules Dosage for Pediatrics
Dabigatran etexilate capsules can be used in pediatric patients aged 8 to less than 18 years of age who are able to swallow the capsules whole. Other age- appropriate pediatric dosage forms of dabigatran etexilate are available for pediatric patients less than 8 years of age. For the treatment of VTE in pediatric patients, initiate treatment following treatment with a parenteral anticoagulant for at least 5 days. For reduction in risk of recurrence of VTE, initiate treatment following previous treatment.
Dabigatran etexilate capsules is dosed orally twice daily, one dose in the morning and one dose in the evening, at approximately the same time every day. The dosing interval should be as close to 12 hours as possible.
The recommended dosage of dabigatran etexilate capsules for the treatment of or reducing the risk of VTE in pediatric patients 8 to less than 18 years of age is based on the patient’s actual weight as shown in Table 1 below. Administer dabigatran etexilate capsules twice daily. Adjust the dosage according to actual weight as treatment progresses [see Dosage and Administration ( 2.5)].
Table 1 Weight-Based Dabigatran Etexilate Capsules Dosage for Pediatric Patients Aged 8 to Less Than 18 Years
|
Actual Weight (kg) |
Dosage (mg) |
Number of Capsules |
|
11 kg to less than 16 kg |
75 mg twice daily |
one 75 mg capsule |
|
16 kg to less than 26 kg |
110 mg twice daily |
one 110 mg capsule |
|
26 kg to less than 41 kg |
150 mg twice daily |
one 150 mg capsule |
|
41 kg to less than 61 kg |
185 mg twice daily |
one 110 mg capsule |
|
61 kg to less than 81 kg |
220 mg twice daily |
two 110 mg capsule twice daily |
|
81 kg or greater |
260 mg twice daily |
one 150 mg capsule plus one 110 mg capsule twice daily |
2.4 Dosage Adjustments
Adult patients with renal impairment
Assess renal function prior to initiation of treatment with dabigatran
etexilate capsules. Periodically assess renal function as clinically indicated
(i.e., more frequently in clinical situations that may be associated with a
decline in renal function) and adjust therapy accordingly. Discontinue
dabigatran etexilate capsules in patients who develop acute renal failure
while on dabigatran etexilate capsules and consider alternative anticoagulant
therapy.
Generally, in adult patients, the extent of anticoagulation does not need to
be assessed. When necessary, use aPTT or ECT, and not INR, to assess for
anticoagulant activity in adult patients on dabigatran etexilate capsules [see Warnings and Precautions ( 5.2) and Clinical Pharmacology ( 12.2)].
Reduction of Risk of Stroke and Systemic Embolism in Non-valvular Atrial
Fibrillation
In patients with moderate renal impairment (CrCl 30 to 50 mL/min), concomitant
use of the P-gp inhibitor dronedarone or systemic ketoconazole can be expected
to produce dabigatran exposure similar to that observed in severe renal
impairment. Reduce the dosage of dabigatran etexilate capsules to 75 mg twice
daily [see Warnings and Precautions ( 5.5), Drug Interactions ( 7.1), and Clinical Pharmacology ( 12.3)].
Treatment and Reduction in the Risk of Recurrence of Deep Venous Thrombosis
and Pulmonary Embolism
Dosing recommendations for patients with CrCl ≤ 30 mL/min cannot be provided.
Avoid use of concomitant P-gp inhibitors in patients with CrCl < 50 mL/min
[see Warnings and Precautions ( 5.5), Drug Interactions ( 7.2) and Clinical Pharmacology ( 12.3)].
Pediatric patients with renal impairment
Prophylaxis of Deep Vein Thrombosis and Pulmonary Embolism Following Hip
Replacement Surgery
Dosing recommendations for patients with CrCl ≤ 30 mL/min or on dialysis
cannot be provided. Avoid use of concomitant P-gp inhibitors in patients with
CrCl < 50 mL/min [see Dosage and Administration ( 2.5), Warnings and Precautions ( 5.5), Drug Interactions ( 7.3), and Clinical Pharmacology ( 12.2, 12.3)].
Treatment and reduction in risk of recurrence of VTE in pediatric patients
Due to lack of data in pediatric patients with eGFR < 50 mL/min/1.73 m 2and the risk of increased exposure, avoid use of dabigatran etexilate capsules in these patients. Prior to the initiation of treatment with dabigatran etexilate capsules, estimate the glomerular filtration rate (eGFR) using the Schwartz formula: eGFR (Schwartz) = (0.413 x height in cm) / serum creatinine in mg/dL.
Treat patients with an eGFR > 50 mL/min/1.73 m2 with the dosage according to Table 1 [see Dosage and Administration ( 2.3)].
2.5 Administration
Dabigatran etexilate capsules should be swallowed whole. Dabigatran etexilate capsules should be taken with a full glass of water. Breaking, chewing, or emptying the contents of the capsule can result in increased exposure [see Clinical Pharmacology ( 12.3)].
If a dose of dabigatran etexilate capsules is not taken at the scheduled time, the dose should be taken as soon as possible on the same day; the missed dose should be skipped if it cannot be taken at least 6 hours before the next scheduled dose. The dose of dabigatran etexilate capsules should not be doubled to make up for a missed dose.
Consideradministration with food if gastrointestinal distress occurs with dabigatran etexilate capsules.
2.6 Converting from or to Warfarin
When converting patients from warfarin therapy to dabigatran etexilate capsules, discontinue warfarin and start dabigatran etexilate capsules when the INR is below 2.0.
When converting from dabigatran etexilate capsules to warfarin, adjust the starting time of warfarin as follows:
Adults
• For CrCl ≥ 50 mL/min, start warfarin 3 days before discontinuing dabigatran
etexilate capsules.
• For CrCl 30 to 50 mL/min, start warfarin 2 days before discontinuing
dabigatran etexilate capsules.
• For CrCl 15 to 30 mL/min, start warfarin 1 day before discontinuing
dabigatran etexilate capsules.
• For CrCl < 15 mL/min, no recommendations can be made.
Pediatrics
• For eGFR ≥ 50 mL/min/1.73 m 2, start warfarin 3 days before discontinuing
dabigatran etexilate capsules.
• Pediatric patients with an eGFR < 50 mL/min/1.73 m 2have not been studied.
Avoid use of dabigatran etexilate capsules in these patients.
Because dabigatran etexilate capsules can increase INR, the INR will better reflect warfarin’s effect only after dabigatran etexilate capsules has been stopped for at least 2 days [see Clinical Pharmacology ( 12.2)].
2.7 Converting from or to Parenteral Anticoagulants
For adult and pediatric patients currently receiving a parenteral
anticoagulant, start dabigatran etexilate capsules 0 to 2 hours before the
time that the next dose of the parenteral drug was to have been administered
or at the time of discontinuation of a continuously administered parenteral
drug (e.g., intravenous unfractionated heparin).
For adult patients currently taking dabigatran etexilate capsules wait 12
hours (CrCl ≥ 30 mL/min) or 24 hours (CrCl < 30 mL/min) after the last dose of
dabigatran etexilate capsules before initiating treatment with a parenteral
anticoagulant [see Clinical Pharmacology ( 12.3)].
For pediatric patients currently taking dabigatran etexilate capsules, wait 12 hours after the last dose before switching to a parenteral anticoagulant.
2.8 Discontinuation for Surgery and Other Interventions
If possible, discontinue dabigatran etexilate capsules in adults 1 to 2 days (CrCl ≥ 50 mL/min) or 3 to 5 days (CrCl < 50 mL/min) before invasive or surgical procedures because of the increased risk of bleeding. Consider longer times for patients undergoing major surgery, spinal puncture, or placement of a spinal or epidural catheter or port, in whom complete hemostasis may be required [see Use in Specific Populations ( 8.6) and Clinical Pharmacology ( 12.3)].
For pediatric patients, discontinue dabigatran etexilate capsules 24 hours before an elective surgery (eGFR > 80 mL/min/1.73 m 2) or 2 days before an elective surgery (eGFR 50 to 80 mL/min/1.73 m 2). Pediatric patients with an eGFR <50 mL/min/1.73 m 2have not been studied, avoid use of dabigatran etexilate capsules in these patients.
If surgery cannot be delayed, there is an increased risk of bleeding [see Warnings and Precautions ( 5.2)]. This risk of bleeding should be weighed against the urgency of intervention [see Warnings and Precautions ( 5.1, 5.3)]. Use a specific reversal agent (idarucizumab) in case of emergency surgery or urgent procedures when reversal of the anticoagulant effect of dabigatran is needed in adults. Efficacy and safety of idarucizumab have not been established in pediatric patients [see Warnings and Precautions ( 5.2)]. Refer to the idarucizumab prescribing information for additional information. Restart dabigatran etexilate capsules as soon as medically appropriate.
• Non-valvular Atrial Fibrillation in Adult Patients:
o For patients with CrCl > 30 mL/min: 150 mg orally, twice daily ( 2.2)
o For patients with CrCl 15 to 30 mL/min: 75 mg orally, twice daily ( 2.2)
• Treatment of DVT and PE in Adult Patients:
o For patients with CrCl > 30 mL/min: 150 mg orally, twice daily after 5 to 10
days of parenteral anticoagulation ( 2.2)
• Reduction in the Risk of Recurrence of DVT and PE in Adult Patients:
o For patients with CrCl > 30 mL/min: 150 mg orally, twice daily after
previous treatment ( 2.2)
• Prophylaxis of DVT and PE Following Hip Replacement Surgery in Adult Patients:
o For patients with CrCl > 30 mL/min: 110 mg orally first day, then 220 mg once daily ( 2.2)
• Treatment of Pediatric VTE:
o For pediatric patients: weight-based dosage, twice daily after at least 5
days of parenteral anticoagulant ( 2.3)
• Reduction in the Risk of Recurrence of Pediatric VTE:
o For pediatric patients: weight-based dosage, twice daily after previous
treatment ( 2.3)
• Dabigatran etexilate capsules are NOT substitutable on a milligram-to-
milligram basis with other dabigatran etexilate dosage forms
• Review recommendations for converting to or from other oral or parenteral
anticoagulants (2.6, 2.7)
• Temporarily discontinue dabigatran etexilate capsules before invasive or
surgical procedures when possible, then restart promptly ( 2.8)
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Dabigatran was not carcinogenic when administered by oral gavage to mice and
rats for up to 2 years. The highest doses tested (200 mg/kg/day) in mice and
rats were approximately 3.6 and 6 times, respectively, the human exposure at
MRHD of 300 mg/day based on AUC comparisons.
Dabigatran was not mutagenic in in vitrotests, including bacterial reversion
tests, mouse lymphoma assay and chromosomal aberration assay in human
lymphocytes, and the in vivomicronucleus assay in rats.
In the rat fertility study with oral gavage doses of 15, 70, and 200 mg/kg,
males were treated for 29 days prior to mating, during mating up to scheduled
termination, and females were treated 15 days prior to mating through
gestation Day 6. No adverse effects on male or female fertility were observed
at 200 mg/kg or 9 to 12 times the human exposure at MRHD of 300 mg/day based
on AUC comparisons. However, the number of implantations decreased in females
receiving 70 mg/kg, or 3 times the human exposure at MRHD based on AUC
comparisons.
OVERDOSAGE SECTION
10 OVERDOSAGE
Accidental overdose may lead to hemorrhagic complications. In the event of hemorrhagic complications, initiate appropriate clinical support, discontinue treatment with dabigatran etexilate capsules, and investigate the source of bleeding. A specific reversal agent (idarucizumab) is available for adult patients.
Dabigatran is primarily eliminated by the kidneys with a low plasma protein binding of approximately 35%. Hemodialysis can remove dabigatran; however, data supporting this approach are limited. Using a high-flux dialyzer, blood flow rate of 200 mL/min, and dialysate flow rate of 700 mL/min, approximately 49% of total dabigatran can be cleared from plasma over 4 hours. At the same dialysate flow rate, approximately 57% can be cleared using a dialyzer blood flow rate of 300 mL/min, with no appreciable increase in clearance observed at higher blood flow rates. Upon cessation of hemodialysis, a redistribution effect of approximately 7% to 15% is seen. The effect of dialysis on dabigatran’s plasma concentration would be expected to vary based on patient specific characteristics. Measurement of aPTT or ECT may help guide therapy [see Warnings and Precautions ( 5.2) and Clinical Pharmacology ( 12.2)].
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Dabigatran etexilate 75 mg capsules are cream opaque cap/cream opaque body
size '2' HPMC capsules imprinted with 'H' on cap and 'D10' on body with black
ink, filled with mixture of off-white to yellowish white pellets. The capsules
are supplied in the packages listed:
Unit of use bottle of 60 capsules NDC 31722-621-60
Blister package containing 60 capsules
(10 x 6 capsule blister cards) NDC 31722-621-32
Dabigatran etexilate 110 mg capsules are cream opaque cap/cream opaque body
size ‘1’ HPMC capsules imprinted with ‘H’ on cap and ‘D16’ on body with black
ink, filled with mixture of off-white to yellowish white pellets. The capsules
are supplied in the packages listed:
Unit of use bottle of 60 capsules NDC 31722-666-60
Blister package containing 60 capsules
(10 x 6 capsule blister cards) NDC 31722-666-32
Dabigatran etexilate 150 mg capsules are cream opaque cap/cream opaque body
size '0' HPMC capsules imprinted with 'H' on cap and 'D11' on body with black
ink, filled with mixture of off-white to yellowish white pellets.
Unit of use bottle of 60 capsules NDC 31722-622-60
Blister package containing 60 capsules (10 x 6 capsule blister cards) NDC 31722-622-32
Bottles
Store at 20°C to 25°C (68°F to 77°F) [see USP Controlled Room Temperature].
Once opened, the product must be used within 4 months. Keep the bottle tightly
closed. Store in the original package to protect from moisture.
Blisters
Store at 20°C to 25°C (68°F to 77°F) [see USP Controlled Room Temperature].
Store in the original package to protect from moisture.
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
150 mg capsules with a cream opaque cap/cream opaque body size ‘0’ HPMC
capsules imprinted with ‘H’ on cap and ‘D11’ on body with black ink, filled
with mixture of off-white to yellowish white pellets.
110 mg capsules with a cream opaque cap/cream opaque body size ‘1’ HPMC
capsules imprinted with ‘H’ on cap and ‘D16’ on body with black ink, filled
with mixture of off white to yellowish white pellets.
75 mg capsules with a cream opaque cap/cream opaque body size ‘2’ HPMC
capsules imprinted with ‘H’ on cap and ‘D10’ on body with black ink, filled
with mixture of off-white to yellowish white pellets.
Capsules: 75 mg, 110 mg and 150 mg ( 3)
SPL MEDGUIDE SECTION
MEDICATION GUIDE
|
** Dabigatran Etexilate** |
|
This Medication Guide is for dabigatran etexilate capsules. |
|
What is the most important information I should know about dabigatran
etexilate capsules? |
|
What are dabigatran etexilate capsules? •in children: It is not known if dabigatran etexilate capsules are safe and effective in children with atrial fibrillation not caused by a heart valve problem, or in children who have undergone hip replacement surgery. |
|
Do not take dabigatran etexilate capsules if you: |
|
Before taking dabigatran etexilate capsules, tell your healthcare provider
about all of your medical conditions, including if you: |
|
How should I take dabigatran etexilate capsules? • In adults: Take dabigatran etexilate capsules 2 times a day. If you are
taking dabigatran etexilate capsules after hip replacement surgery, take
dabigatran etexilate capsules 1 time a day. • You can take dabigatran etexilate capsules with or without food. Taking
dabigatran etexilate capsules with food may help if you have an upset stomach.
• Swallow dabigatran etexilate capsules whole with a full glass of water. Tell
your healthcare provider if you or your child are not able to swallow the
capsules whole. Do not break, chew, or empty the pellets from the capsule. |
|
What are the possible side effects of dabigatran etexilate capsules? ◾ hives ◾ rash ◾ itching |
|
How should I store dabigatran etexilate capsules? |
|
General information about the safe and effective use of dabigatran
etexilate****capsules |
|
What are the ingredients in dabigatran etexilate capsules? The brands listed are trademarks of their respective owners and are not
trademarks of Hetero Labs Limited.
Manufactured for: Manufactured by: |
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Revised: 07/2025
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
The limited available data on dabigatran etexilate capsules use in pregnant
women are insufficient to determine drug-associated risks for adverse
developmental outcomes. There are risks to the mother associated with
untreated venous thromboembolism in pregnancy and a risk of hemorrhage in the
mother and fetus associated with the use of anticoagulants (see Clinical
Considerations).In pregnant rats treated from implantation until weaning,
dabigatran increased the number of dead offspring and caused excess
vaginal/uterine bleeding close to parturition at an exposure 2.6 times the
human exposure. At a similar exposure, dabigatran decreased the number of
implantations when rats were treated prior to mating and up to implantation
(gestation Day 6). Dabigatran administered to pregnant rats and rabbits during
organogenesis up to exposures 8 and 13 times the human exposure, respectively,
did not induce major malformations. However, the incidence of delayed or
irregular ossification of fetal skull bones and vertebrae was increased in the
rat (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
Pregnancy confers an increased risk for thromboembolism that is higher for
women with underlying thromboembolic disease and certain high-risk pregnancy
conditions. Published data describe that women with a previous history of
venous thrombosis are at high risk for recurrence during pregnancy.
Fetal/Neonatal adverse reaction
Use of anticoagulants, including dabigatran etexilate capsules, may increase
the risk of bleeding in the fetus and neonate. Monitor neonates for bleeding
[see Warnings and Precautions ( 5.2)].
Labor or delivery
All patients receiving anticoagulants, including pregnant women, are at risk
for bleeding. Dabigatran etexilate capsules use during labor or delivery in
women who are receiving neuraxial anesthesia may result in epidural or spinal
hematomas. Consider discontinuation or use of shorter acting anticoagulant as
delivery approaches [see Warnings and Precautions ( 5.2, 5.3)].
Data
Animal Data
Dabigatran has been shown to decrease the number of implantations when male
and female rats were treated at a dosage of 70 mg/kg (about 2.6 to 3.0 times
the human exposure at MRHD of 300 mg/day based on area under the curve [AUC]
comparisons) prior to mating and up to implantation (gestation Day 6).
Treatment of pregnant rats after implantation with dabigatran at the same dose
increased the number of dead offspring and caused excess vaginal/uterine
bleeding close to parturition. Dabigatran administered to pregnant rats and
rabbits during organogenesis up to maternally toxic doses of 200 mg/kg (8 and
13 times the human exposure, respectively, at a MRHD of 300 mg/day based on
AUC comparisons) did not induce major malformations, but increased the
incidence of delayed or irregular ossification of fetal skull bones and
vertebrae in the rat.
Death of offspring and mother rats during labor in association with uterine
bleeding occurred during treatment of pregnant rats from implantation
(gestation Day 7) to weaning (lactation Day 21) with dabigatran at a dose of
70 mg/kg (about 2.6 times the human exposure at MRHD of 300 mg/day based on
AUC comparisons).
8.2 Lactation
Risk Summary
There are insufficient data to assess the presence of dabigatran in human milk. There are no data on the effects of dabigatran on the breastfed child or on milk production. Dabigatran and/or its metabolites were present in rat milk. Breastfeeding is not recommended during treatment with dabigatran etexilate capsules.
8.3 Females and Males of Reproductive Potential
Females of reproductive potential requiring anticoagulation should discuss
pregnancy planning with their physician.
The risk of clinically significant uterine bleeding, potentially requiring
gynecological surgical interventions, identified with oral anticoagulants
including dabigatran etexilate capsules should be assessed in females of
reproductive potential and those with abnormal uterine bleeding.
8.4 Pediatric Use
The safety and effectiveness of dabigatran etexilate capsules for the treatment and the reduction in risk of recurrence of venous thromboembolism have been established in pediatric patients 8 to less than 18 years of age. Use of dabigatran etexilate for this indication is supported by evidence from adequate and well-controlled studies in pediatric patients. These studies included an open-label, randomized, parallel-group study and an open-label, single-arm safety study [see Adverse Reactions ( 6.1) and Clinical Studies ( 14.4, 14.5)] . Other age-appropriate pediatric dosage forms of dabigatran etexilate are available for pediatric patients less than 8 years of age for these indications.
Safety and effectiveness of dabigatran etexilate capsules have not been established in pediatric patients with non-valvular atrial fibrillation or those who have undergone hip replacement surgery.
8.5 Geriatric Use
Of the total number of patients in the RE-LY study, 82% were 65 and over, while 40% were 75 and over. The risk of stroke and bleeding increases with age, but the risk-benefit profile is favorable in all age groups [see Warnings and Precautions ( 5), Adverse Reactions ( 6.1), and Clinical Studies ( 14.1)].
8.6 Renal Impairment
Reduction of Risk of Stroke and Systemic Embolism in Non-valvular Atrial
Fibrillation in Adult Patients
No dose adjustment of dabigatran etexilate capsules is recommended in patients
with mild or moderate renal impairment [see Clinical Pharmacology ( 12.3)].
Reduce the dose of dabigatran etexilate capsules in patients with severe renal
impairment (CrCl 15 to 30 mL/min) [see Dosage and Administration ( 2.2, 2.4) and Clinical Pharmacology ( 12.3)]. Dosing recommendations for patients with
CrCl < 15 mL/min or on dialysis cannot be provided.
Adjust dose appropriately in patients with renal impairment receiving concomitant P-gp inhibitors [see Warnings and Precautions ( 5.5), Drug Interactions ( 7.1), and Clinical Pharmacology ( 12.3)].
Treatment and Reduction in the Risk of Recurrence of Deep Venous Thrombosis
and Pulmonary Embolism in Adult Patients
Patients with severe renal impairment (CrCl ≤ 30 mL/min) were excluded from
RE-COVER.
Dosing recommendations for patients with CrCl ≤ 30 mL/min or on dialysis cannot be provided. Avoid use of dabigatran etexilate capsules with concomitant P-gp inhibitors in patients with CrCl < 50 mL/min [see Warnings and Precautions ( 5.5), Drug Interactions ( 7.2), and Clinical Pharmacology ( 12.3)].
Prophylaxis of Deep Vein Thrombosis and Pulmonary Embolism in Adult Patients
Following Hip Replacement Surgery
Patients with severe renal impairment (CrCl < 30 mL/min) were excluded from
RE-NOVATE and RE-NOVATE II.
Dosing recommendations for patients with CrCl < 30 mL/min or on dialysis
cannot be provided.
Avoid use of dabigatran etexilate capsules with concomitant P-gp inhibitors in patients with CrCl <50 mL/min [see Warnings and Precautions ( 5.5), Drug Interactions (7.3), and Clinical Pharmacology ( 12.2, 12.3)].
Treatment and Reduction in the Risk of Recurrence of VTE in Pediatric Patients
Dabigatran etexilate has not been studied in pediatric patients with eGFR <50 mL/min/1.73 m 2. Reduced renal function could increase exposure. Dosing recommendations cannot be provided for treatment of these patients. Avoid use of dabigatran etexilate capsules in these patients [see Dosage and Administration ( 2.4)].
• Lactation: Breastfeeding not recommended ( 8.2)
• Geriatric Use: Risk of bleeding increases with age ( 8.5)
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Dabigatran and its acyl glucuronides are competitive, direct thrombin inhibitors. Because thrombin (serine protease) enables the conversion of fibrinogen into fibrin during the coagulation cascade, its inhibition prevents the development of a thrombus. Both free and clot-bound thrombin, and thrombin-induced platelet aggregation are inhibited by the active moieties.
12.2 Pharmacodynamics
At recommended therapeutic doses, dabigatran etexilate prolongs the
coagulation markers such as aPTT, ECT, TT, and dTT. INR is relatively
insensitive to the exposure to dabigatran and cannot be interpreted the same
way as used for warfarin monitoring.
Adults
The aPTT test provides an approximation of dabigatran etexilate capsules
anticoagulant effect. The average time course for effects on aPTT, following
approved dosing regimens in patients with various degrees of renal impairment
is shown in Figure 2. The curves represent mean levels without confidence
intervals; variations should be expected when measuring aPTT. While advice
cannot be provided on the level of recovery of aPTT needed in any particular
clinical setting, the curves can be used to estimate the time to get to a
particular level of recovery, even when the time since the last dose of
dabigatran etexilate capsules is not precisely known. In the RE-LY trial, the
median (10 thto 90 thpercentile) trough aPTT in patients receiving the 150 mg
dose was 52 (40 to 76) seconds.
Figure 2 Average Time Course for Effects of Dabigatran on aPTT, Following
Approved Dabigatran Etexilate Capsules Dosing Regimens in Adult Patients with
Various Degrees of Renal Impairment*
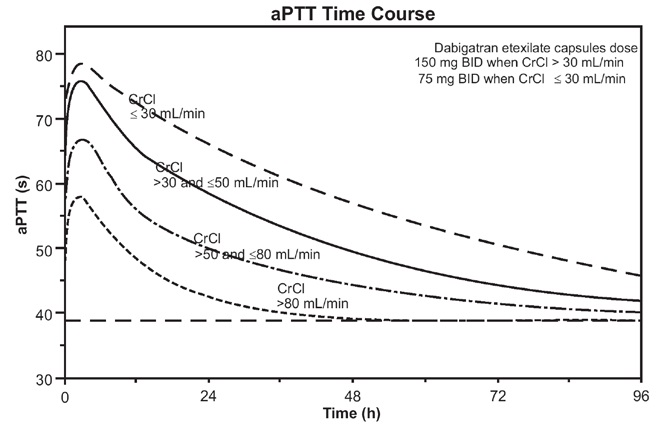
*Simulations based on PK data from a study in subjects with renal impairment and PK/aPTT relationships derived from the RE-LY study; aPTT prolongation in RE-LY was measured centrally in citrate plasma using PTT Reagent Roche Diagnostics GmbH, Mannheim, Germany. There may be quantitative differences between various established methods for aPTT assessment.
The degree of anticoagulant activity can also be assessed by the ecarin clotting time (ECT). This test is a more specific measure of the effect of dabigatran than activated partial thromboplastin time (aPTT). In the RE-LY trial, the median (10 thto 90 thpercentile) trough ECT in patients receiving the 150 mg dose was 63 (44 to 103) seconds.
In orthopedic hip surgery patients, maximum aPTT response (E max) to dabigatran and baseline aPTT were higher shortly after surgery than at later time points (e.g. ≥3 days after surgery).
Pediatrics
As in adults, there is a correlation between plasma dabigatran concentrations and the degree of its anticoagulant effect in pediatric patients with venous thromboembolism. The parameters dTT and ECT increased in direct linear proportion to the plasma concentration of dabigatran, whereas aPTT prolongation increases in a nonlinear fashion with dabigatran plasma concentrations.
Similar PK/PD relationships for aPTT, ECT, and dTT were observed across age groups of pediatric patients (ages 26 days to < 18 years) and between pediatric and adult patients with venous thromboembolism. This similarity in PK/PD relationship suggests that similar exposure-response relationship is expected for dabigatran etexilate treatment across the pediatric age groups and adult patients.
Cardiac Electrophysiology
No prolongation of the QTc interval was observed with dabigatran etexilate at
doses up to 600 mg.
12.3 Pharmacokinetics
Dabigatran etexilate mesylate is absorbed as the dabigatran etexilate ester.
The ester is then hydrolyzed, forming dabigatran, the active moiety.
Dabigatran is metabolized to four different acyl glucuronides and both the
glucuronides and dabigatran have similar pharmacological activity.
Pharmacokinetics described here refer to the sum of dabigatran and its
glucuronides. Dabigatran displays dose-proportional pharmacokinetics in
healthy adult subjects and adult patients in the range of doses from 10 to 400
mg. Given twice daily, dabigatran’s accumulation factor in adults and
pediatrics is approximately two.
Absorption
The absolute bioavailability of dabigatran following oral administration of
dabigatran etexilate is approximately 3% to 7%. Dabigatran etexilate is a
substrate of the efflux transporter P-gp. After oral administration of
dabigatran etexilate in healthy volunteers, C maxoccurs at 1-hour post-
administration in the fasted state. Coadministration of dabigatran etexilate
capsules with a high-fat meal delays the time to C maxby approximately 2 hours
but has no effect on the bioavailability of dabigatran; dabigatran etexilate
capsules may be administered with or without food.
The oral bioavailability of dabigatran etexilate increases by 75% when the
pellets are taken without the capsule shell compared to the intact capsule
formulation based on a single-dose relative bioavailability study. Dabigatran
etexilate capsules should therefore not be broken, chewed, or opened before
administration.
Dabigatran etexilate is available in capsules and oral pellets. The approved indications and intended age groups are not the same. Oral absorption of dabigatran etexilate is formulation-dependent. At steady-state, dabigatran etexilate oral pellets show 37% higher relative bioavailability in healthy adults compared to dabigatran etexilate capsules based on a multiple-dose relative bioavailability study. In addition, the relative bioavailability between the two dosage forms is age-dependent. The relative bioavailability observed in adults cannot be translated to pediatrics.
Distribution
Dabigatran is approximately 35% bound to human plasma proteins. The red blood
cell to plasma partitioning of dabigatran measured as total radioactivity is
less than 0.3. The volume of distribution of dabigatran is 50 to 70 L.
Elimination
Dabigatran is eliminated primarily in the urine. Renal clearance of dabigatran
is 80% of total clearance after intravenous administration. After oral
administration of radiolabeled dabigatran, 7% of radioactivity is recovered in
urine and 86% in feces. The half-life of dabigatran in healthy adult subjects
is 12 to 17 hours. Population pharmacokinetic simulation shows that the
elimination half-life in pediatric patients is 12 to 14 hours.
Metabolism
After oral administration, dabigatran etexilate is converted to dabigatran.
The cleavage of the dabigatran etexilate by esterase-catalyzed hydrolysis to
the active principal dabigatran is the predominant metabolic reaction.
Dabigatran is not a substrate, inhibitor, or inducer of CYP450 enzymes.
Dabigatran is subject to conjugation, forming pharmacologically active acyl
glucuronides. Four positional isomers, 1-O, 2-O, 3-O, and 4-O-acylglucuronide
exist, and each accounts for less than 10% of total dabigatran in plasma.
Specific Populations
Pediatric Patients
The pharmacokinetics of dabigatran was characterized in two clinical studies (DIVERSITY and Study 2) following multiple doses in pediatric patients from birth to less than 18 years old. In pediatric patients taking age- and weight- adjusted dosages of dabigatran etexilate capsules (aged 8-18 years), the observed geometric mean steady-state trough concentration was 97.9 ng/mL (63.7 to 151 ng/mL, 10 thto 90 thpercentile) compared to the steady-state geometric mean trough concentration of 59.7 ng/mL (26.3 to 146 ng/mL, 10 thto 90 thpercentile) observed in adult patients with DVT/PE.
Renal Impairment
An open, parallel-group, single-center study compared dabigatran pharmacokinetics in healthy adult subjects and adult patients with mild to moderate renal impairment receiving a single dose of dabigatran etexilate capsules 150 mg. Exposure to dabigatran increases with severity of renal function impairment (Table 10). Similar findings were observed in the RE-LY, RE-COVER and RE-NOVATE II trials.
Table 10 Impact of Renal Impairment on Dabigatran Pharmacokinetics
|
Renal Function |
CrCl |
Increase in |
Increase in |
t****1/2 |
|
Normal |
≥ 80 |
1x |
1x |
13 |
|
Mild |
50 to 80 |
1.5x |
1.1x |
15 |
|
Moderate |
30 to 50 |
3.2x |
1.7x |
18 |
|
Severe**+** |
15 to 30 |
6.3x |
2.1x |
27 |
+Patients with severe renal impairment were not studied in RE-LY, RE-COVER and
RE-NOVATE II. Dosing recommendations in subjects with severe renal impairment
are based on pharmacokinetic modeling [see Dosage and Administration ( 2.2, 2.4) and Use in Specific Populations ( 8.6)].
Hepatic Impairment
Administration of dabigatran etexilate capsules in adult patients with
moderate hepatic impairment (Child-Pugh B) showed a large inter-subject
variability, but no evidence of a consistent change in exposure or
pharmacodynamics.
Drug Interactions
A summary of the effect of coadministered drugs on dabigatran exposure in
healthy adult subjects is shown in Figures 3.1 and 3.2.
In the orthopedic hip surgery patients, limited clinical data with P-gp inhibitors is available.
Figure 3.1 Effect of P-gp Inhibitor or Inducer (rifampicin) Drugs on Peak and Total Exposure to Dabigatran (Cmax and AUC). Shown are the Geometric Mean Ratios (Ratio) and 90% Confidence Interval (90% CI). The Perpetrator and Dabigatran Etexilate Dosage and**Dosage**Frequency are given as well as the Time of Perpetrator**Dosage**in Relation to Dabigatran EtexilateDosage****(Time Difference)
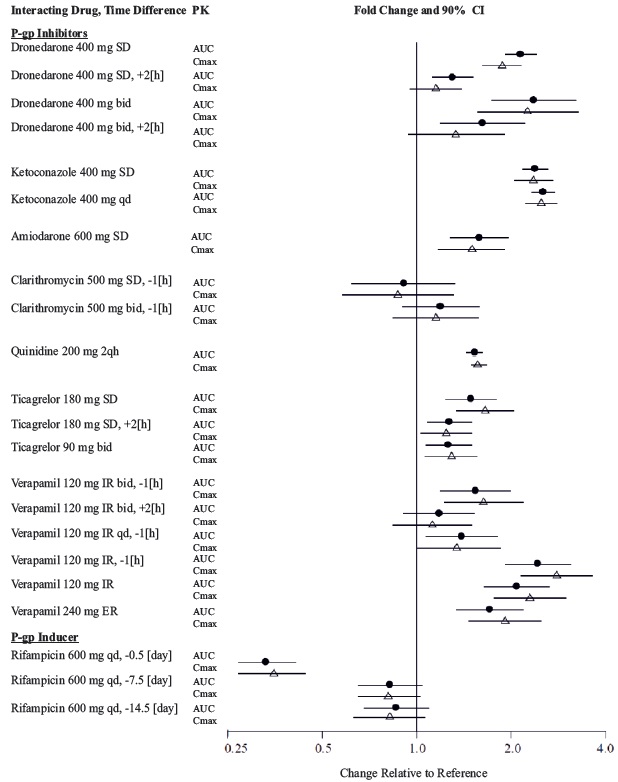
Figure 3.2 Effect of Non-P-gp Inhibitor or Inducer, Other Drugs, on Peak and Total Exposure to Dabigatran (Cmaxand AUC). Shown are the Geometric Mean Ratios (Ratio) and 90% Confidence Interval (90% CI). The Perpetrator and Dabigatran Etexilate**Dosage**and**Dosage**Frequency are given as well as the Time of Perpetrator**Dosage**in Relation to Dabigatran EtexilateDosage****(Time Difference)
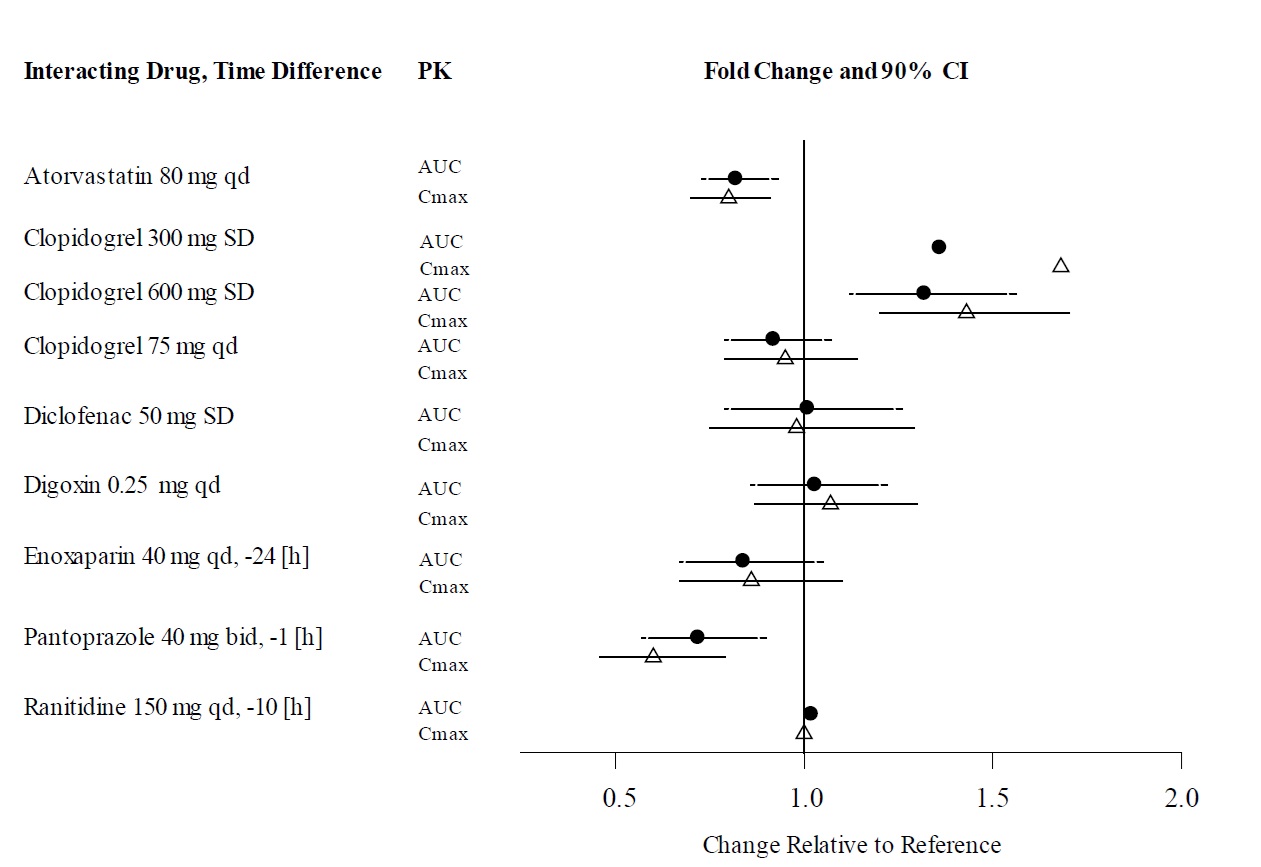
In RE-LY, dabigatran plasma samples were also collected. The concomitant use
of proton pump inhibitors, H2 antagonists, and digoxin did not appreciably
change the trough concentration of dabigatran.
Impact of Dabigatran on Other Drugs
In clinical studies exploring CYP3A4, CYP2C9, P-gp and other pathways,
dabigatran did not meaningfully alter the pharmacokinetics of amiodarone,
atorvastatin, clarithromycin, diclofenac, clopidogrel, digoxin, pantoprazole,
or ranitidine.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Reduction of Risk of Stroke and Systemic Embolism in Non-valvular
Atrial Fibrillation in Adult Patients
The clinical evidence for the efficacy of dabigatran etexilate capsules was
derived from RE-LY (Randomized Evaluation of Long-term Anticoagulant Therapy),
a multi-center, multi-national, randomized, parallel group trial comparing two
blinded dosages of dabigatran etexilate capsules (110 mg twice daily and 150
mg twice daily) with open-label warfarin (dosed to target INR of 2 to 3) in
patients with non-valvular, persistent, paroxysmal, or permanent atrial
fibrillation and one or more of the following additional risk factors:
• Previous stroke, transient ischemic attack (TIA), or systemic embolism
• Left ventricular ejection fraction < 40%
• Symptomatic heart failure, ≥ New York Heart Association Class 2
• Age ≥ 75 years
• Age ≥ 65 years and one of the following: diabetes mellitus, coronary artery
disease (CAD), or hypertension
The primary objective of this study was to determine if dabigatran etexilate
capsules was non-inferior to warfarin in reducing the occurrence of the
composite endpoint, stroke (ischemic and hemorrhagic) and systemic embolism.
The study was designed to ensure that dabigatran etexilate capsules preserved
more than 50% of warfarin’s effect as established by previous randomized,
placebo-controlled trials of warfarin in atrial fibrillation. Statistical
superiority was also analyzed.
A total of 18,113 patients were randomized and followed for a median of 2
years. The patients’ mean age was 71.5 years and the mean CHADS 2score was
2.1. The patient population was 64% male, 70% Caucasian, 16% Asian, and 1%
black. Twenty percent of patients had a history of a stroke or TIA and 50%
were vitamin K antagonist (VKA) naïve, defined as less than 2 months total
lifetime exposure to a VKA. Thirty-two percent of the population had never
been exposed to a VKA. Concomitant diseases of patients in this trial included
hypertension 79%, diabetes 23%, and CAD 28%. At baseline, 40% of patients were
on aspirin and 6% were on clopidogrel. For patients randomized to warfarin,
the mean percentage of time in therapeutic range (INR 2 to 3) was 64%.
Relative to warfarin and to dabigatran etexilate capsules 110 mg twice daily,
dabigatran etexilate capsules 150 mg twice daily significantly reduced the
primary composite endpoint of stroke and systemic embolism (see Table 11 and
Figure 4).
**Table 11 First Occurrence of Stroke or Systemic Embolism in the RE-LY Study
|
Dabigatran Etexilate Capsules |
Dabigatran Etexilate Capsules |
Warfarin | |
|
Patients randomized |
6,076 |
6,015 |
6,022 |
|
Patients (% per yr) with events |
135 (1.12%) |
183 (1.54%) |
203 (1.72%) |
|
Hazard ratio vs warfarin (95% CI) |
0.65 (0.52, 0.81) |
0.89 (0.73, 1.09) | |
|
P-value for superiority |
0.0001 |
0.27 | |
|
Hazard ratio vs dabigatran etexilate capsules 110 mg (95% CI) |
0.72 (0.58, 0.91) | ||
|
P-value for superiority |
0.005 |
*Randomized ITT
Figure 4 Kaplan-Meier Curve Estimate of Time to First Stroke or Systemic
Embolism
The contributions of the components of the composite endpoint, including
stroke by subtype, are shown in Table 12. The treatment effect was primarily a
reduction in stroke. Dabigatran etexilate capsules 150 mg twice daily was
superior in reducing ischemic and hemorrhagic strokes relative to warfarin.
Table 12 Strokes and Systemic Embolism in the RE-LY Study
|
Dabigatran EtexilateCapsules150 mg twice daily |
Warfarin |
Hazard****ratio vs warfarin (95% CI) | |
|
Patients randomized |
6,076 |
6,022 | |
|
Stroke |
123 |
187 |
0.64 (0.51, 0.81) |
|
Ischemic stroke |
104 |
134 |
0.76 (0.59, 0.98) |
|
Hemorrhagic stroke |
12 |
45 |
0.26 (0.14, 0.49) |
|
Systemic embolism |
13 |
21 |
0.61 (0.30, 1.21) |
In the RE-LY trial, the rate of all-cause mortality was lower on dabigatran
etexilate capsules 150 mg than on warfarin (3.6% per year versus 4.1% per
year). The rate of vascular death was lower on dabigatran etexilate capsules
150 mg compared to warfarin (2.3% per year versus 2.7% per year). Non-vascular
death rates were similar in the treatment arms.
The efficacy of dabigatran etexilate capsules 150 mg twice daily was generally
consistent across major subgroups (see Figure 5).
Figure 5 Stroke and Systemic Embolism Hazard Ratios by Baseline
Characteristics*****
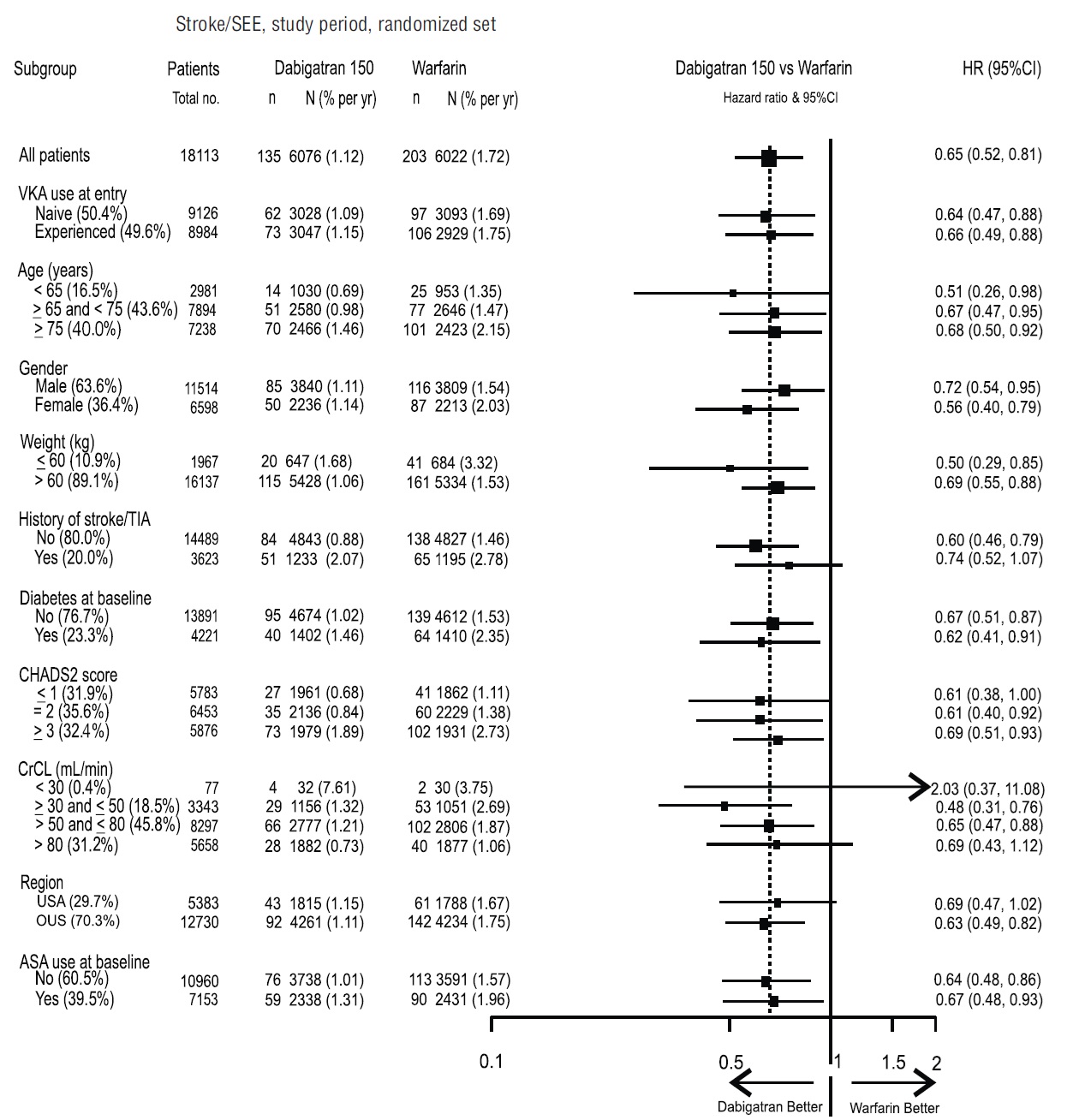
*Randomized ITT
Note: The figure above presents effects in various subgroups all of which are
baseline characteristics and all of which were pre-specified. The 95%
confidence limits that are shown do not take into account how many comparisons
were made, nor do they reflect the effect of a particular factor after
adjustment for all other factors. Apparent homogeneity or heterogeneity among
groups should not be over-interpreted.
In RE-LY, a higher rate of clinical myocardial infarction was reported in
patients who received dabigatran etexilate capsules (0.7 per 100 patient-years
for 150 mg dose) than in those who received warfarin (0.6).
14.2 Treatment and Reduction in the Risk of Recurrence of Deep Venous
Thrombosis and Pulmonary Embolism in Adult Patients
In the randomized, parallel group, double-blind trials, RE-COVER and RE-COVER
II, patients with deep vein thrombosis and pulmonary embolism received
dabigatran etexilate capsules 150 mg twice daily or warfarin (dosed to target
INR of 2 to 3) following initial treatment with an approved parenteral
anticoagulant for 5 to 10 days.
In RE-COVER, the median treatment duration during the oral only treatment
period was 174 days. A total of 2,539 patients (30.9% patients with
symptomatic PE with or without DVT and 68.9% with symptomatic DVT only) were
treated with a mean age of 54.7 years. The patient population was 58.4% male,
94.8% white, 2.6% Asian, and 2.6% black. The concomitant diseases of patients
in this trial included hypertension (35.9%), diabetes mellitus (8.3%),
coronary artery disease (6.5%), active cancer (4.8%), and gastric or duodenal
ulcer (4.4%). Concomitant medications included agents acting on renin-
angiotensin system (25.2%), vasodilators (28.4%), serum lipid-reducing agents
(18.2%), NSAIDs (21%), beta-blockers (14.8%), calcium channel blockers (8.5%),
ASA (8.6%), and platelet inhibitors excluding ASA (0.6%). Patients randomized
to warfarin had a mean percentage of time in the INR target range of 2.0 to
3.0 of 60% in RE-COVER study.
In RE-COVER II, the median treatment duration during the oral only treatment
period was 174 days. A total of 2,568 patients (31.8% patients with
symptomatic PE with or without DVT and 68.1% with symptomatic DVT only) were
treated with a mean age of 54.9 years. The patient population was 60.6% male,
77.6% white, 20.9% Asian, and 1.5% black. The concomitant diseases of patients
in this trial included hypertension (35.1%), diabetes mellitus (9.8%),
coronary artery disease (7.1%), active cancer (3.9%), and gastric or duodenal
ulcer (3.8%). Concomitant medications included agents acting on renin-
angiotensin system (24.2%), vasodilators (28.6%), serum lipid-reducing agents
(20.0%), NSAIDs (22.3%), beta-blockers (14.8%), calcium channel blockers
(10.8%), ASA (9.8%), and platelet inhibitors excluding ASA (0.8%). Patients
randomized to warfarin had a mean percentage of time in the INR target range
of 2.0 to 3.0 of 57% in RE-COVER II study.
In studies RE-COVER and RE-COVER II, the protocol specified non-inferiority
margin (2.75) for the hazard ratio was derived based on the upper limit of the
95% confidence interval of the historical warfarin effect. Dabigatran
etexilate capsules was demonstrated to be non-inferior to warfarin (dosed to
target INR of 2 to 3) (Table 13) based on the primary composite endpoint
(fatal PE or symptomatic non-fatal PE and/or DVT) and retains at least 66.9%
(RE-COVER) and 63.9% (RE-COVER II) of the historical warfarin effect,
respectively.
Table 13 Primary Efficacy Endpoint for RE-COVER and RE-COVER II – Modified
ITTaPopulation
|
Dabigatran EtexilateCapsules |
Warfarin |
Hazard****ratio vs warfarin (95% CI) | |
|
RE-COVER |
N=1,274 |
N=1,265 | |
|
Primary Composite Endpoint b |
34 (2.7) |
32 (2.5) |
1.05 (0.65, 1.70) |
|
Fatal PE c |
1 (0.1) |
3 (0.2) | |
|
Symptomatic non-fatal PE c |
16 (1.3) |
8 (0.6) | |
|
Symptomatic recurrent DVT c |
17 (1.3) |
23 (1.8) | |
|
RE-COVER II |
N=1,279 |
N=1,289 | |
|
Primary Composite Endpoint b |
34 (2.7) |
30 (2.3) |
1.13 (0.69, 1.85) |
|
Fatal PE c |
3 (0.2) |
0 | |
|
Symptomatic non-fatal PE c |
9 (0.7) |
15 (1.2) | |
|
Symptomatic recurrent DVT c |
30 (2.3) |
17 (1.3) |
aModified ITT analyses population consists of all randomized patients who
received at least one dose of study medication.
bNumber of patients with one or more event.
cNumber of events. For patients with multiple events each event is counted
independently.
In the randomized, parallel-group, double-blind, pivotal trial, RE-MEDY,
patients received dabigatran etexilate capsules 150 mg twice daily or warfarin
(dosed to target INR of 2 to 3) following 3 to 12 months of treatment with
anticoagulation therapy for an acute VTE. The median treatment duration during
the treatment period was 534 days. A total of 2,856 patients were treated with
a mean age of 54.6 years. The patient population was 61% male, and 90.1%
white, 7.9% Asian and 2.0% black. The concomitant diseases of patients in this
trial included hypertension (38.6%), diabetes mellitus (9.0%), coronary artery
disease (7.2%), active cancer (4.2%), and gastric or duodenal ulcer (3.8%).
Concomitant medications included agents acting on renin-angiotensin system
(27.9%), vasodilators (26.7%), serum lipid reducing agents (20.6%), NSAIDs
(18.3%), beta-blockers (16.3%), calcium channel blockers (11.1%), aspirin
(7.7%), and platelet inhibitors excluding ASA (0.9%). Patients randomized to
warfarin had a mean percentage of time in the INR target range of 2.0 to 3.0
of 62% in the study.
In study RE-MEDY, the protocol specified non-inferiority margin (2.85) for the
hazard ratio was derived based on the point estimate of the historical
warfarin effect. Dabigatran etexilate capsules were demonstrated to be non-
inferior to warfarin (dosed to target INR of 2 to 3) (Table 14) based on the
primary composite endpoint (fatal PE or symptomatic non-fatal PE and/or DVT)
and retains at least 63.0% of the historical warfarin effect. If the non-
inferiority margin was derived based on the 50% retention of the upper limit
of the 95% confidence interval, dabigatran etexilate capsules was demonstrated
to retain at least 33.4% of the historical warfarin effect based on the
composite primary endpoint.
Table 14 Primary Efficacy Endpoint for RE-MEDY - Modified ITT
aPopulation
|
Dabigatran EtexilateCapsules |
Warfarin |
Hazard****ratio vs warfarin | |
|
Primary Composite Endpoint b |
26 (1.8) |
18 (1.3) |
1.44 (0.78, 2.64) |
|
Fatal PE c |
1 (0.07) |
1 (0.07) | |
|
Symptomatic non-fatal PE c |
10 (0.7) |
5 (0.4) | |
|
Symptomatic recurrent DVT c |
17 (1.2) |
13 (0.9) |
aModified ITT analyses population consists of all randomized patients who
received at least one dose of study medication.
bNumber of patients with one or more event.
cNumber of events. For patients with multiple events each event is counted
independently.
In a randomized, parallel-group, double-blind, pivotal trial, RE-SONATE,
patients received dabigatran etexilate capsules 150 mg twice daily or placebo
following 6 to 18 months of treatment with anticoagulation therapy for an
acute VTE. The median treatment duration was 182 days. A total of 1,343
patients were treated with a mean age of 55.8 years. The patient population
was 55.5% male, 89.0% white, 9.3% Asian, and 1.7% black. The concomitant
diseases of patients in this trial included hypertension (38.8%), diabetes
mellitus (8.0%), coronary artery disease (6.0%), history of cancer (6.0%),
gastric or duodenal ulcer (4.5%), and heart failure (4.6%). Concomitant
medications included agents acting on renin-angiotensin system (28.7%),
vasodilators (19.4%), beta-blockers (18.5%), serum lipid reducing agents
(17.9%), NSAIDs (12.1%), calcium channel blockers (8.9%), aspirin (8.3%), and
platelet inhibitors excluding ASA (0.7%). Based on the outcome of the primary
composite endpoint (fatal PE, unexplained death, or symptomatic non-fatal PE
and/or DVT), dabigatran etexilate capsules was superior to placebo (Table 15).
Table 15 Primary Efficacy Endpoint for RE-SONATE - Modified ITT
aPopulation
|
Dabigatran EtexilateCapsules |
Placebo |
Hazard****ratio vs placebo | |
|
Primary Composite Endpoint b |
3 (0.4) |
37 (5.6) |
0.08 (0.02, 0.25) |
|
Fatal PE and unexplained death c |
0 |
2 (0.3) | |
|
Symptomatic non-fatal PE c |
1 (0.1) |
14 (2.1) | |
|
Symptomatic recurrent DVT c |
2 (0.3) |
23 (3.5) |
aModified ITT analyses population consists of all randomized patients who
received at least one dose of study medication.
bNumber of patients with one or more events.
cNumber of events. For patients with multiple events each event is counted
independently.
14.3 Prophylaxis of Deep Vein Thrombosis and Pulmonary Embolism in Adult
Patients Following Hip Replacement Surgery
In the randomized, parallel-group, double-blind, non-inferiority trials, RE-
NOVATE and RE-NOVATE II patients received dabigatran etexilate capsules 75 mg
orally 1 to 4 hours after surgery followed by 150 mg daily (RE-NOVATE),
dabigatran etexilate capsules 110 mg orally 1 to 4 hours after surgery
followed by 220 mg daily (RE-NOVATE and RE-NOVATE II) or subcutaneous
enoxaparin 40 mg once daily initiated the evening before surgery (RE-NOVATE
and RE-NOVATE II) for the prophylaxis of deep vein thrombosis and pulmonary
embolism in patients who have undergone hip replacement surgery.
Overall, in RE-NOVATE and RE-NOVATE II, the median treatment duration was 33
days for dabigatran etexilate capsules and 33 days for enoxaparin. A total of
5,428 patients were treated with a mean age of 63.2 years. The patient
population was 45.3% male, 96.1% white, 3.6% Asian, and 0.4 % black. The
concomitant diseases of patients in these trials included hypertension
(46.1%), venous insufficiency (15.4%), coronary artery disease (8.2%),
diabetes mellitus (7.9%), reduced renal function (5.3%), heart failure (3.4%),
gastric or duodenal ulcer (3.0%), VTE (2.7%), and malignancy (0.1%).
Concomitant medications included cardiac therapy (69.7%), NSAIDs (68%),
vasoprotectives (29.7%), agents acting on renin-angiotensin system (29.1%),
beta-blockers (21.5%), diuretics (20.8%), lipid modifying agents (18.2%), any
antithrombin/anticoagulant (16.0%), calcium channel blockers (13.6%), low
molecular weight heparin (7.8%), aspirin (7.0%), platelet inhibitors excluding
ASA (6.9%), other antihypertensives (6.7%), and peripheral vasodilators
(2.6%).
For efficacy evaluation all patients were to have bilateral venography of the
lower extremities at 3 days after last dose of study drug unless an endpoint
event had occurred earlier in the study. In the primary efficacy analysis,
dabigatran etexilate capsules 110 mg orally 1 to 4 hours after surgery
followed by 220 mg daily was non-inferior to enoxaparin 40 mg once daily in a
composite endpoint of confirmed VTE (proximal or distal DVT on venogram,
confirmed symptomatic DVT, or confirmed PE) and all cause death during the
treatment period (Tables 16 and 17). In the studies 2628 (76.5%) patients in
RE-NOVATE and 1572 (78.9%) patients in RE-NOVATE II had evaluable venograms at
study completion.
Table 16 Primary Efficacy Endpoint for RE-NOVATE
|
Dabigatran EtexilateCapsules |
Enoxaparin | |
|
Number of Patients****a |
N=880 |
N= 897 |
|
Primary Composite Endpoint |
53 (6.0) |
60 (6.7) |
|
Risk difference (%) vs enoxaparin (95% CI) |
-0.7 (-2.9, 1.6) | |
|
Number of Patients |
N=909 |
N=917 |
|
Composite endpoint of major VTE band VTE related mortality |
28 (3.1) |
36 (3.9) |
|
Number of Patients |
N=905 |
N=914 |
|
Proximal DVT |
23 (2.5) |
33 (3.6) |
|
Number of Patients |
N=874 |
N=894 |
|
Total DVT |
46 (5.3) |
57 (6.4) |
|
Number of Patients |
N=1,137 |
N=1,142 |
|
Symptomatic DVT |
6 (0.5) |
1 (0.1) |
|
PE |
5 (0.4) |
3 (0.3) |
|
Death |
3 (0.3) |
0 |
aFull Analysis Set (FAS): The FAS included all randomized patients who
received at least one subcutaneous injection or one oral dose of study
medication, underwent surgery and subjects for whom the presence or absence of
an efficacy outcome at the end of the study was known, i.e., an evaluable
negative venogram for both distal and proximal DVT in both legs or any of the
following: positive venography in one or both legs, or confirmed symptomatic
DVT, PE, or death during the treatment period.
bVTE is defined as proximal DVT and PE****
** Table 17 Primary Efficacy Endpoint for RE-NOVATE II**
|
Dabigatran EtexilateCapsules |
Enoxaparin | |
|
Number of Patients****a |
N=792 |
N= 786 |
|
Primary Composite Endpoint |
61 (7.7) |
69 (8.8) |
|
Risk difference (%) vs enoxaparin (95% CI) |
-1.1 (-3.8, 1.6) | |
|
Number of Patients |
N=805 |
N=795 |
|
Composite endpoint of major VTE band VTE related mortality |
18 (2.2) |
33 (4.2) |
|
Number of Patients |
N=804 |
N=793 |
|
Proximal DVT |
17 (2.1) |
31 (3.9) |
|
Number of Patients |
N=791 |
N=784 |
|
Total DVT |
60 (7.6) |
67 (8.5) |
|
Number of Patients |
N=1,001 |
N=992 |
|
Symptomatic DVT |
0 |
4 (0.4) |
|
PE |
1 (0.1) |
2 (0.2) |
|
Death |
0 |
1 (0.1) |
aFull Analysis Set (FAS): The FAS included all randomized patients who
received at least one subcutaneous injection or one oral dose of study
medication, underwent surgery and subjects for whom the presence or absence of
an efficacy outcome at the end of the study was known, i.e., an evaluable
negative venogram for both distal and proximal DVT in both legs or any of the
following: positive venography in one or both legs, or confirmed symptomatic
DVT, PE, or death during the treatment period.
bVTE is defined as proximal DVT and PE
14.4 Treatment of VTE in Pediatric Patients
The DIVERSITY study was conducted to demonstrate the efficacy and safety of dabigatran etexilate compared to standard of care (SOC) for the treatment of venous thromboembolism (VTE) in pediatric patients from birth to less than 18 years of age. The study was designed as an open-label, randomized, parallel- group, non-inferiority study. Patients enrolled were randomized according to a 2:1 scheme to either an age-appropriate formulation (capsules, oral pellets, or oral solution) of dabigatran etexilate (doses adjusted for age and weight) after at least 5 days and no longer than 21 days of treatment with a parenteral anticoagulant, or to SOC comprised of low molecular weight heparins (LMWH) or vitamin K antagonists (VKA) or fondaparinux. For patients on dabigatran etexilate, drug concentration was determined prior to the 7 thdose and a single titration was permitted to achieve drug target levels of 50 to 250 ng/mL. Inability to achieve target, after one up-titration, resulted in premature termination of study drug in 12 patients (6.8%).
The median treatment duration during the treatment period was 85 days. In total, 267 patients entered the study (leading index VTE was 64% deep vein thrombosis, 10% cerebral venous thrombosis or sinus thrombosis, and 9.0% pulmonary embolism), with 18% of patients having a central line-associated thrombosis. The patient population was 49.8% male, 91.8% white, 4.9% Asian, and 1.5% black; 168 patients were 12 to < 18 years old, 64 patients 2 to < 12 years, and 35 patients were younger than 2 years. The concomitant VTE-related risk factors of patients in this trial among study arms were as follows: inherited thrombophilia disorder (dabigatran etexilate: 20%, SOC: 22%), congenital heart disease (dabigatran etexilate: 12%, SOC: 30%), heart failure (dabigatran etexilate: 3%, SOC: 18%), history of cancer (dabigatran etexilate: 10%, SOC: 1%), CVL insertion (dabigatran etexilate: 23%, SOC: 27%), immobility (dabigatran etexilate: 13%, SOC: 10%) and significant infection (dabigatran etexilate: 15%, SOC: 13%). The number of patients taking concomitant medications with hemostatic effects were similar in both treatment groups (dabigatran etexilate: 15%, SOC: 16%).
The efficacy of dabigatran etexilate was established based on a composite endpoint of patients with complete thrombus resolution, freedom from recurrent venous thromboembolic event, and freedom from mortality related to venous thromboembolic event (composite primary endpoint). Of the 267 randomized patients, 81 patients (45.8%) in the dabigatran etexilate group and 38 patients (42.2%) in the SOC group met the criteria for the composite primary endpoint. The corresponding rate difference and 95% CI was -0.038 (-0.161, 0.086) and thus demonstrated non-inferiority of dabigatran etexilate to SOC, since the upper bound of the 95% CI was lower than the predefined non- inferiority margin of 20% (see Table 18).
Table 18: Efficacy Results [ITT population] DIVERSITY Study
|
DABIGATRAN ETEXILATE |
Standard of Care | |
|
Number of patients randomized (%) |
177 (100.0) |
90 (100.0) |
|
Complete thrombus resolution |
81 (45.8) |
38 (42.2) |
|
Freedom from recurrent VTE |
170 (96.0) |
83 (92.2) |
|
Freedom from mortality related to VTE |
177 (100.0) |
89 (98.9) |
|
Composite endpoint met |
81 (45.8) |
38 (42.2) |
|
Difference in rate (95% CI) 1 |
-0.038 (-0.161, 0.086) | |
|
p-value for non-inferiority |
< 0.0001 | |
|
p-value for superiority |
0.2739 |
1Mantel-Haenszel weighted difference with age group as stratification factor
Subgroup analyses showed that there were no outliers in the treatment effect for the subgroups by age, sex, region, and presence of certain risk factors (central venous line, congenital heart disease, malignant disease). For the 3 different age strata, the proportions of patients that met the efficacy endpoint in the dabigatran etexilate and SOC groups, respectively, were 13/22 (59.1%) and 7/13 (53.8%) for patients from birth to < 2 years [Rate Difference -0.052; (95%CI: -0.393, 0.288)], 21/43 (48.8%) and 12/21 (57.1%) for patients aged 2 to < 12 years [Rate Difference 0.083; (95%CI: -0.176, 0.342)], and 47/112 (42.0%) and 19/56 (33.9%) for patients aged 12 to < 18 years [Rate Difference - 0.080; (95%CI: -0.234, 0.074)].****
14.5 Reduction in the Risk of Recurrence of VTE in Pediatric Patients
Study 2 was an open-label, single-arm safety study to assess the safety of dabigatran etexilate for the prevention of recurrent VTE in pediatric patients from birth to < 18 years. Patients who required further anticoagulation due to the presence of a clinical risk factor after completing the initial treatment for confirmed VTE (for at least 3 months) or after completing the DIVERSITY study were included in the study. Eligible patients received age- and weight adjusted dosages of an age-appropriate formulation (capsules or oral pellets) of dabigatran etexilate until the clinical risk factor resolved, or up to a maximum of 12 months. The primary endpoints of the study included the recurrence of VTE, major and minor bleeding events, and mortality (overall and related to thrombotic or thromboembolic events) at 6 and 12 months.
Of the 214 patients in the study, 162 patients were 12 to < 18 years old, 43 patients were 2 to < 12 years old, and 9 patients were aged 6 months to < 2 years old.
The overall probability of being free from recurrence of VTE during the on- treatment period was 0.990 (95% CI: 0.960, 0.997) at 3 months, 0.984 (95% CI: 0.950, 0.995) at 6 months, and 0.984 (95% CI: 0.950, 0.995) at 12 months. The probability of being free from bleeding events during the on-treatment period was 0.849 (95% CI: 0.792, 0.891) at 3 months, 0.785 (95% CI: 0.718, 0.838) at 6 months, and 0.723 (95% CI: 0.645, 0.787) at 12 months. No on-treatment deaths occurred.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient or caregiver to read the FDA-approved patient labeling (Medication Guide).
Instructions for Patients
• Tell patients to take dabigatran etexilate capsules exactly as prescribed.
• Remind patients not to discontinue dabigatran etexilate capsules without
talking to the healthcare provider who prescribed it.
• Keep dabigatran etexilate capsules in the original bottle to protect from
moisture. Do not put dabigatran etexilate capsules in pill boxes or pill
organizers.
• When more than one bottle is dispensed to the patient, instruct them to open
only one bottle at a time.
• Instruct patient to remove only one capsule from the opened bottle at the
time of use. The bottle should be immediately and tightly closed.
• Advise patients not to chew or break the capsules before swallowing them and
not to open the capsules and take the pellets alone.
• Advise patients that the capsule should be taken with a full glass of water.
[see Boxed Warning, Dosage and Administration ( 2.5)]
Bleeding
Inform patients that they may bleed more easily, may bleed longer, and should
call their healthcare provider for any signs or symptoms of bleeding [see Warnings and Precautions ( 5.2)].
Instruct patients to seek emergency care right away if they have any of the
following, which may be a sign or symptom of serious bleeding:
• Unusual bruising (bruises that appear without known cause or that get
bigger)
• Pink or brown urine
• Red or black, tarry stools
• Coughing up blood
• Vomiting blood, or vomit that looks like coffee grounds
Instruct patients to call their healthcare provider or to get prompt medical
attention if they experience any signs or symptoms of bleeding:
• Pain, swelling or discomfort in a joint
• Headaches, dizziness, or weakness
• Reoccurring nose bleeds
• Unusual bleeding from gums
• Bleeding from a cut that takes a long time to stop
• Menstrual bleeding or vaginal bleeding that is heavier than normal
If patients have had neuraxial anesthesia or spinal puncture, and particularly, if they are taking concomitant NSAIDs or platelet inhibitors, advise patients to watch for signs and symptoms of spinal or epidural hematoma, such as back pain, tingling, numbness (especially in the lower limbs), muscle weakness, and stool or urine incontinence. If any of these symptoms occur, advise the patient to contact his or her physician immediately [see Boxed Warning].
Gastrointestinal Adverse Reactions
Instruct patients to call their healthcare provider if they experience any
signs or symptoms of dyspepsia or gastritis:
• Dyspepsia (upset stomach), burning, or nausea
• Abdominal pain or discomfort
• Epigastric discomfort, GERD (gastric indigestion)
[see Adverse Reactions ( 6.1)]
Invasive or Surgical Procedures
Instruct patients to inform their healthcare provider that they are taking
dabigatran etexilate capsules before any invasive procedure (including dental
procedures) is scheduled [see Dosage and Administration ( 2.8)].
Concomitant Medications
Ask patients to list all prescription medications, over-the-counter
medications, or dietary supplements they are taking or plan to take so their
healthcare provider knows about other treatments that may affect bleeding risk
(e.g., aspirin or NSAIDs) or dabigatran exposure (e.g., dronedarone or
systemic ketoconazole) [see Warnings and Precautions ( 5.2, 5.5)].
Prosthetic Heart Valves
Instruct patients to inform their healthcare provider if they will have or
have had surgery to place a prosthetic heart valve [see Warnings and Precautions ( 5.4)].
Allergic Reactions
Advise adult patients and caregivers that some adults taking dabigatran
etexilate capsules have developed symptoms of an allergic reaction. Advise
adult patients or caregivers to inform their healthcare provider if they or
their child develop symptoms of an allergic reaction, such as hives, rash, or
itching. Advise adult patients or caregivers to seek emergency medical
attention if they or their child develop chest pain or tightness, swelling of
the face or tongue, trouble breathing or wheezing, or feeling dizzy or faint.
Pregnancy
Advise patients to inform their healthcare provider immediately if they become
pregnant or intend to become pregnant during treatment with dabigatran
etexilate capsules [see Use in Specific Populations ( 8.1)].
Lactation
Advise patients not to breastfeed if they are taking dabigatran etexilate
capsules [see Use in Specific Populations ( 8.2)].

Manufactured for:
Camber Pharmaceuticals, Inc.,
Piscataway, NJ 08854
Manufactured by:
HETERO****TM
Hetero Labs Limited
Jeedimetla, Hyderabad - 500 055,
Telangana, India.
Revised: 07/2025

