Diclofenac sodium
Diclofenac Sodium 2% Topical Solution
fc0e1950-d76f-75bc-e053-6294a90a8c12
HUMAN PRESCRIPTION DRUG LABEL
May 22, 2023
Advanced Rx Pharmacy of Tennessee, LLC
DUNS: 117023142
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Diclofenac sodium
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL

OVERDOSAGE SECTION
10 OVERDOSAGE
Symptoms following acute NSAID overdosages have been typically limited to lethargy, drowsiness, nausea, vomiting, and epigastric pain, which have been generally reversible with supportive care. Gastrointestinal bleeding has occurred. Hypertension, acute renal failure, respiratory depression, and coma have occurred but were rare [ see Warnings and Precautions ( 5.1, 5.2, 5.4, 5.6) ].
Manage patients with symptomatic and supportive care following an NSAID overdosage. There are no specific antidotes. Emesis is not recommended due to a possibility of aspiration and subsequent respiratory irritation by DMSO contained in diclofenac sodium topical solution. Consider activated charcoal (60 to 100 grams in adults, 1 to 2 grams per kg of body weight in pediatric patients) and/or osmotic cathartic in symptomatic patients seen within four hours of ingestion or in patients with a large overdosage (5 to 10 times the recommended dosage). Forced diuresis, alkalinization of urine, hemodialysis, or hemoperfusion may not be useful due to high protein binding.
For additional information about overdosage treatment, contact a poison control center (1-800-222-1222).
DESCRIPTION SECTION
11 DESCRIPTION
Diclofenac sodium topical solution USP, 2% w/w, contains diclofenac sodium, USP a benzeneacetic acid derivative that is a nonsteroidal anti-inflammatory drug, and is available as a clear, colorless to faintly pink or orange solution for topical application. The chemical name is 2 [(2,6-dichlorophenyl) amino]-benzeneacetic acid, monosodium salt. The molecular weight is 318.14. Its molecular formula is C 14H 10Cl 2NNaO 2, and it has the following chemical structure.
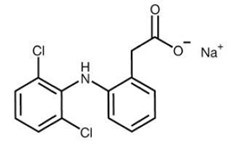
Each 1 gram of solution contains 20 mg of diclofenac sodium, USP. The inactive ingredients: dimethyl sulfoxide USP (DMSO, 45.5% w/w), ethanol (31.3% v/v), purified water, propylene glycol, and hydroxypropyl cellulose.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Diclofenac has analgesic, anti-inflammatory, and antipyretic properties.
The mechanism of action of diclofenac sodium topical solution, like that of other NSAIDs, is not completely understood but involves inhibition of cyclooxygenase (COX-1 and COX-2).
Diclofenac is a potent inhibitor of prostaglandin synthesis in vitro. Diclofenac concentrations reached during therapy have produced in vivo effects. Prostaglandins sensitize afferent nerves and potentiate the action of bradykinin in inducing pain in animal models. Prostaglandins are mediators of inflammation. Because diclofenac is an inhibitor of prostaglandin synthesis, its mode of action may be due to a decrease of prostaglandins in peripheral tissues.
12.3 Pharmacokinetics
Absorption:
After administration of diclofenac sodium topical solution (40 mg/knee every 12 h; total daily diclofenac exposure: 80 mg/knee) for 7.5 days, the mean (SD) AUC 0-12 and mean (SD) C max were 77.27 (49.89) ng∙h/mL and 12.16 (7.66) ng/mL, respectively, on Day 1; and 204.58 (111.02) ng∙h/mL and 25.24 (12.95) ng/mL, respectively, at steady state on Day 8. After administration of diclofenac sodium topical solution 1.5% (19.3 mg/knee every 6 h; total daily diclofenac exposure 77.2 mg/knee), the mean (SD) AUC 0- 12 and mean (SD) C max were 27.46 (23.97) ng∙h/mL and 2.30 (2.02) ng/mL, respectively, on Day 1; and 141.49 (92.47) ng∙h/mL and 17.04 (11.28) ng/mL, respectively, at steady state on Day 8.
The pharmacokinetics and effect of diclofenac sodium topical solution were not evaluated under the conditions of heat application, occlusive dressings overlay, or exercise following product application. Therefore, concurrent use of diclofenac sodium topical solution under these conditions is not recommended.
Distribution:
Diclofenac is more than 99% bound to human serum proteins, primarily to albumin
Diclofenac diffuses into and out of the synovial fluid. Diffusion into the joint occurs when plasma levels are higher than those in the synovial fluid, after which the process reverses and synovial fluid levels are higher than plasma levels. It is not known whether diffusion into the joint plays a role in the effectiveness of diclofenac.
Elimination
Metabolism:
Five diclofenac metabolites have been identified in human plasma and urine. The metabolites include 4'-hydroxy-, 5-hydroxy-, 3'-hydroxy-, 4',5-dihydroxy- and 3'-hydroxy-4'-methoxy diclofenac. The major diclofenac metabolite, 4'-hydroxy-diclofenac, has very weak pharmacologic activity. The formation of 4'-hydroxy diclofenac is primarily mediated by CYP2C9. Both diclofenac and its oxidative metabolites undergo glucuronidation or sulfation followed by biliary excretion. Acylglucuronidation mediated by UGT2B7 and oxidation mediated by CYP2C8 may also play a role in diclofenac metabolism. CYP3A4 is responsible for the formation of minor metabolites, 5-hydroxy and 3'-hydroxy-diclofenac.
Excretion:
Diclofenac is eliminated through metabolism and subsequent urinary and biliary excretion of the glucuronide and the sulfate conjugates of the metabolites.
Little or no free unchanged diclofenac is excreted in the urine.
Specific Populations:
Pediatric: The pharmacokinetics of diclofenac sodium topical solution has not been investigated in pediatric patients.
Race: Pharmacokinetic differences due to race have not been studied.
Drug Interaction Studies
Aspirin: When NSAIDs were administered with aspirin, the protein binding of NSAIDs were reduced, although the clearance of free NSAID was not altered. The clinical significance of this interaction is not known. See Table 3 for clinically significant drug interactions of NSAIDs with aspirin [ see Drug Interactions ( 7) ].
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Carcinogenicity studies in mice and rats administered diclofenac sodium as a dietary constituent for 2 years resulted in no significant increases in tumor incidence at doses up to 2 mg/kg/day approximately 0.85 and 1.7 times, respectively, the maximum recommended human topical dose of diclofenac sodium topical solution (based on apparent bioavailability and body surface area comparison).
In a dermal carcinogenicity study conducted in albino mice, daily topical applications of diclofenac sodium for two years at concentrations up to 0.035% diclofenac sodium (a 57-fold lower diclofenac sodium concentration than present in diclofenac sodium topical solution) did not increase neoplasm incidence.
In a photococarcinogenicity study conducted in hairless mice, topical application of diclofenac sodium at doses up to 0.035% diclofenac sodium (a 57-fold lower diclofenac sodium concentration than present in diclofenac sodium topical solution) resulted in an earlier median time of onset of tumors.
Mutagenesis
Diclofenac was not mutagenic or clastogenic in a battery of genotoxicity tests that included the bacterial reverse mutation assay, in vitro mouse lymphoma point mutation assay, chromosomal aberration studies in Chinese hamster ovarian cells in vitro, and in vivo rat chromosomal aberration assay of bone marrow cells.
Impairment of Fertility
Fertility studies have not been conducted with diclofenac sodium topical solution. Diclofenac sodium administered to male and female rats at doses up to 4 mg/kg/day (approximately 3.4 times the MRHD of diclofenac sodium topical solution based on apparent bioavailability and body surface area comparison) did not affect fertility. Studies conducted in rats found no effect of dermally applied DMSO on fertility, reproductive performance, or offspring performance.
However, published studies report that treatment of adult male rats with diclofenac by the oral route at 10 mg/kg (0.6 times the MRHD) for 14 days and at 0.25 mg/kg (0.01 times the MRHD) for 30 days produced adverse effects on male reproductive hormones and testes.
13.2 Animal Toxicology and/or Pharmacology
Ocular Effects
No adverse effects were observed using indirect ophthalmoscopy after multiple- daily dermal application to rats for 26 weeks and minipigs for 52 weeks of DMSO at twice the concentration found in diclofenac sodium topical solution. Published studies of dermal or oral administration of DMSO to rabbits, dogs and pigs described refractive changes of lens curvature and cortical fibers indicative of myopic changes and/or incidences of lens opacity or discoloration when evaluated using slit-lamp biomicroscopy examination, although no ocular abnormalities were observed in rhesus monkeys during daily oral or dermal treatment with DMSO for 9 to 18 months.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Study in Osteoarthritis of the Knee
Diclofenac sodium topical solution
The use of diclofenac sodium topical solution for the treatment of pain of osteoarthritis of the knee was evaluated in a single double-blind controlled trial conducted in the US, involving patients treated with diclofenac sodium topical solution at a dose of 2 pumps twice a day for 4 weeks. Diclofenac sodium topical solution was compared to topical vehicle, applied directly to the study knee. In this trial, patients treated with diclofenac sodium topical solution experienced a greater reduction in the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain subscale compared to patients treated with vehicle. Numerical results of the WOMAC pain subscale are summarized in Table 4.
Table 4: Change in Treatment Outcomes after 4 Weeks of Treatment with Diclofenac Sodium Topical Solution
| ||
|
** Efficacy Variable** |
** Treatment** | |
|
** Diclofenac Sodium Topical Solution** |
** Vehicle Control** | |
|
WOMAC Pain Subscale * |
12.4 |
`12.6 |
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Diclofenac sodium topical solution USP, 2% w/w, is supplied as a clear, colorless to faintly pink or orange solution containing 20 mg of diclofenac sodium per gram of solution, in a white polypropylene-dose pump bottle with a clear cap. Each pump actuation delivers 20 mg of diclofenac sodium in 1 gram of solution.
NDC Number & Size
112 g bottle NDC:80425-0337-01
Storage
Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide) that accompanies each prescription dispensed. Inform patients, families, or their caregivers of the following information before initiating therapy with diclofenac sodium topical solution and periodically during the course of ongoing therapy.
Cardiovascular Thrombotic Events
Advise patients to be alert for the symptoms of cardiovascular thrombotic events, including chest pain, shortness of breath, weakness, or slurring of speech, and to report any of these symptoms to their health care provider immediately [see Warnings and Precautions ( 5.1)].
Gastrointestinal Bleeding, Ulceration, and Perforation
Advise patients to report symptoms of ulceration and bleeding, including epigastric pain, dyspepsia, melena, and hematemesis to their health care provider. In the setting of concomitant use of low-dose aspirin for cardiac prophylaxis, inform patients of the increased risk for and the signs and symptoms of GI bleeding [see Warnings and Precautions ( 5.2)] .
Hepatotoxicity
Inform patients of the warning signs and symptoms of hepatotoxicity (e.g., nausea, fatigue, lethargy, pruritus, jaundice, right upper quadrant tenderness, and "flu-like" symptoms). If these occur, instruct patients to stop diclofenac sodium topical solution and seek immediate medical therapy [see Warnings and Precautions ( 5.3)] .
Heart Failure and Edema
Advise patients to be alert for the symptoms of congestive heart failure including shortness of breath, unexplained weight gain, or edema and to contact their healthcare provider if such symptoms occur [see Warnings and Precautions ( 5.5)] .
Anaphylactic Reactions
Inform patients of the signs of an anaphylactic reaction (e.g., difficulty breathing, swelling of the face or throat). Instruct patients to seek immediate emergency help if these occur [see Contraindications ( 4) and Warnings and Precautions ( 5.7)] .
Serious Skin Reactions, including DRESS
Advise patients to stop using diclofenac sodium topical solution immediately if they develop any type of rash or fever and contact their health care provider as soon as possible [ seeWarnings and Precautions ( 5.9, 5.10) ].
Female Fertility
Advise females of reproductive potential who desire pregnancy that NSAIDs, including diclofenac sodium topical solution, may be associated with a reversible delay in ovulation [see Use in Specific Populations ( 8.3)]
Fetal Toxicity
Inform pregnant women to avoid use of diclofenac sodium topical solution and other NSAIDs starting at 30 weeks gestation because of the risk of the premature closing of the fetal ductus arteriosus [see Warnings and Precautions ( 5.11)] . If treatment with diclofenac sodium topical solution is needed for a pregnant woman between about 20 to 30 weeks gestation, advise her that she may need to be monitored for oligohydramnios, if treatment continues for longer than 48 hours [see Warnings and Precautions ( 5.11) and Use in Specific Populations ( 8.1)] .
Avoid Concomitant Use of NSAIDs
Inform patients that the concomitant use of diclofenac sodium topical solution with other NSAIDs or salicylates (e.g.,diflunisal, salsalate) is not recommended due to the increased risk of gastrointestinal toxicity, and little or no increase in efficacy [see Warnings and Precautions ( 5.2) and Drug Interactions ( 7)] . Alert patients that NSAIDs may be present in "over the counter" medications for treatment of colds, fever, or insomnia.
Use of NSAIDs and Low-Dose Aspirin
Inform patients not to use low-dose aspirin concomitantly with diclofenac sodium topical solution until they talk to their healthcare provider [see Drug Interactions ( 7)] .
Eye Exposure
Instruct patients to avoid contact of diclofenac sodium topical solution with the eyes and mucosa. Advise patients that if eye contact occurs, immediately wash out the eye with water or saline and consult a physician if irritation persists for more than an hour.
Prevention of Secondary Exposure
Instruct patients to avoid skin-to-skin contact between other people and the knee(s) to which diclofenac sodium topical solution was applied until the knee(s) is completely dry.
Special Application Instructions
Instruct patients not to apply diclofenac sodium topical solution to open skin wounds, infections, inflammations, or exfoliative dermatitis, as it may affect absorption and reduce tolerability of the drug.
Instruct patients to wait until the area treated with diclofenac sodium topical solution is completely dry before applying sunscreen, insect repellant, lotion, moisturizer, cosmetics, or other topical medication.
Instruct patients to minimize or avoid exposure of treated knee(s) to natural or artificial sunlight.
Manufactured for:
Alembic Pharmaceuticals, Inc.
Bedminster, NJ 07921, USA
Manufactured by:
Alembic Pharmaceuticals Limited
(Derma Division),
Karakhadi, Vadodara 391450, India
Mfg. License no.: G/25/2216
Revised: 12/2022
Distributed by:
Advanced Rx Pharmacy of Tennessee, LLC
