Meclizine Hydrochloride
These highlights do not include all the information needed to use MECLIZINE HYDROCHLORIDE TABLETS safely and effectively. See full prescribing information for MECLIZINE HYDROCHLORIDE TABLETS. MECLIZINE HYDROCHLORIDE tablets, for oral use Initial U.S. Approval: 1957
af1e45ca-1478-4eeb-8fdc-4f3d3eda4ed5
HUMAN PRESCRIPTION DRUG LABEL
Jun 23, 2025
American Health Packaging
DUNS: 929561009
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Meclizine Hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
Meclizine Hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Package/Label Display Panel – Blister – 25 mg

Meclizine
Hydrochloride
Tablet, USP
25 mg
DESCRIPTION SECTION
11 DESCRIPTION
Meclizine hydrochloride, a histamine (H1) receptor antagonist, is a white or slightly yellowish, crystalline powder. Its molecular formula is C 25H 27ClN 2•2HCl•H 2O and its molecular weight is 481.88. It has the following structural formula:
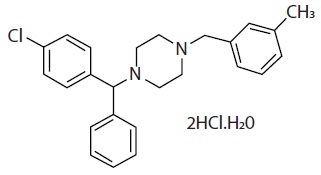
Chemically, meclizine hydrochloride is 1-( p-chloro-α-phenylbenzyl)-4-( m-methylbenzyl) piperazine dihydrochloride monohydrate.
Each meclizine hydrochloride 12.5 mg tablet contains 12.5 mg of meclizine dihydrochloride equivalent to 10.53 mg of meclizine free base.
Each meclizine hydrochloride 25 mg tablet contains 25 mg of meclizine dihydrochloride equivalent to 21.07 mg of meclizine free base.
Inactive ingredients for the tablets are: colloidal silicon dioxide, lactose monohydrate, magnesium stearate, microcrystalline cellulose, sodium starch glycolate and talc. The 12.5 mg tablets also contain FD&C Blue #1 Aluminum Lake. The 25 mg tablets also contain D&C Yellow #10 Aluminum Lake.
SPL UNCLASSIFIED SECTION
PACKAGING INFORMATION
American Health Packaging unit dose blisters (see How Supplied section)
contain drug product from Amneal Pharmaceuticals LLC as follows:
(12.5 mg / 100 UD) NDC 60687-775-01 packaged from NDC 53746-441
(12.5 mg / 50 UD) NDC 60687-775-65 packaged from NDC 53746-441
(25 mg / 100 UD) NDC 60687-730-01 packaged from NDC 53746-442
(25 mg / 50 UD) NDC 60687-730-65 packaged from NDC 53746-442
Distributed by:
American Health Packaging
Columbus, OH 43217
8473001/1224(F)
