Nalfon
These highlights do not include all the information needed to use NALFON safely and effectively. See full prescribing information for NALFON. NALFON (fenoprofen calcium, USP) capsules, for oral use Initial U.S. Approval: 1982
12266b6d-8a6d-425b-88f9-8c6b4d5b1850
HUMAN PRESCRIPTION DRUG LABEL
Apr 1, 2022
Proficient Rx LP
DUNS: 079196022
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
fenoprofen calcium
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 400 mg
NDC 63187-706-90-Rx Only
NALFON**®**
(fenoprofen calcium, USP) capsules
400 mg
Manufactured for:
Xspire Pharma
Ridgeland, MS. 39157
Relabeled by:
Proficient Rx LP
Thousand Oaks, CA 91320
90 capsules

Boxed Warning section
WARNING: RISK OF SERIOUS CARDIOVASCULAR AND GASTROINTESTINAL EVENTS
See full prescribing information for complete boxed warning
•
**Non-Steroidal Anti-Inflammatory drugs (NSAIDs) cause an increased risk of serious cardiovascular thrombotic events, including myocardial infarction and stroke, which can be fatal. This risk may occur early in treatment and may increase with duration of use. (****5.1****)**
•
**NALFON is contraindicated in the setting of coronary artery bypass graft (CABG) surgery (****4****,****5.1****)**
•
**NSAIDs cause an increased risk of serious gastrointestinal (GI) adverse events including bleeding, ulceration, and perforation of stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients and patients with a prior history of peptic ulcer disease and/or GI bleeding are at greater risk for serious GI events (****5.2****)**
INDICATIONS & USAGE SECTION
1. INDICATIONS AND USAGE
NALFON is indicated for:
•
Relief of mild to moderate pain in adults.
•
Relief of the signs and symptoms of rheumatoid arthrites.
•
Relief of the signs and symptoms of osteoarthritis.
NALFON is a nonsteroidal anti-inflammatory drug indicated for:
•
Relief of mild to moderate pain in adults. ( 1)
•
Relief of the signs and symptoms of rheumatoid arthritis. ( 1)
•
Relief of the signs and symptoms of osteoarthritis. ( 1)
CONTRAINDICATIONS SECTION
4. CONTRAINDICATIONS
NALFON is contraindicated in the following patients:
•
Known hypersensitivity (e.g., anaphylactic reactions and serious skin reactions) to fenoprofen or any components of the drug product [ see Warnings and Precautions ( 5.7, 5.9) ]
•
History of asthma, urticaria, or other allergic-type reactions after taking aspirin or other NSAIDs. Severe, sometimes fatal, anaphylactic reactions to NSAIDs have been reported in such patients [ see Warnings and Precautions ( 5.7, 5.8) ]
•
In the setting of coronary artery bypass graft (CABG) surgery [ see Warnings and Precautions ( 5.1) ]
•
Known hypersensitivity to fenoprofen or any components of the drug product ( 4)
•
History of asthma, urticaria, or other allergic-type reactions after taking aspirin or other NSAIDs ( 4)
•
In the setting of CABG surgery ( 4)
ADVERSE REACTIONS SECTION
6. ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the labeling:
•
Cardiovascular Thrombotic Events [ see Warnings and Precautions ( 5.1) ]
•
GI Bleeding, Ulceration and Perforation [ see Warnings and Precautions ( 5.2) ]
•
Hepatotoxicity [ see Warnings and Precautions ( 5.3) ]
•
Hypertension [ see Warnings and Precautions ( 5.4) ]
•
Heart Failure and Edema [ see Warnings and Precautions ( 5.5) ]
•
Renal Toxicity and Hyperkalemia [ see Warnings and Precautions ( 5.6) ]
•
Anaphylactic Reactions [ see Warnings and Precautions ( 5.7) ]
•
Serious Skin Reactions [ see Warnings and Precautions ( 5.9) ]
•
Hematologic Toxicity [ see Warnings and Precautions ( 5.11) ]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
During clinical studies for rheumatoid arthritis, osteoarthritis, or mild to moderate pain and studies of pharmacokinetics, complaints were compiled from a checklist of potential adverse reactions, and the following data emerged. These encompass observations in 6,786 patients, including 188 observed for at least 52 weeks. For comparison, data are also presented from complaints received from the 266 patients who received placebo in these same trials. During short-term studies for analgesia, the incidence of adverse reactions was markedly lower than that seen in longer-term studies.
Adverse Drug Reactions Reported in >1% of Patients During Clinical Trials
Digestive System — During clinical trials with Nalfon, the most common adverse reactions were gastrointestinal in nature and occurred in 20.8% of patients receiving Nalfon as compared to 16.9% of patients receiving placebo. In descending order of frequency, these reactions included dyspepsia (10.3% Nalfon vs. 2.3% placebo), nausea (7.7% vs. 7.1%), constipation (7% vs. 1.5%), vomiting (2.6% vs. 1.9%), abdominal pain (2% vs. 1.1%), and diarrhea (1.8% vs. 4.1%). The drug was discontinued because of adverse gastrointestinal reactions in less than 2% of patients during premarketing studies.
Nervous System — The most frequent adverse neurologic reactions were headache (8.7% vs. 7.5%) and somnolence (8.5% vs. 6.4%). Dizziness (6.5% vs. 5.6%), tremor (2.2% vs. 0.4%), and confusion (1.4% vs. none) were noted less frequently. Nalfon was discontinued in less than 0.5% of patients because of these side effects during premarketing studies.
Skin and Appendages— Increased sweating (4.6% vs. 0.4%), pruritus (4.2% vs. 0.8%), and rash (3.7% vs. 0.4%) were reported. Nalfon was discontinued in about 1% of patients because of an adverse effect related to the skin during premarketing studies.
Special Senses — Tinnitus (4.5% vs. 0.4%), blurred vision (2.2% vs. none), and decreased hearing (1.6% vs. none) were reported. Nalfon was discontinued in less than 0.5% of patients because of adverse effects related to the special senses during premarketing studies.
Cardiovascular — Palpitations (2.5% vs. 0.4%). Nalfon was discontinued in about 0.5% of patients because of adverse cardiovascular reactions during premarketing studies.
Miscellaneous — Nervousness (5.7% vs. 1.5%), asthenia (5.4% vs. 0.4%), peripheral edema (5.0% vs. 0.4%), dyspnea (2.8% vs. none), fatigue (1.7% vs. 1.5%), upper respiratory infection (1.5% vs. 5.6%), and nasopharyngitis (1.2% vs. none).
Adverse Drug Reactions Reported in <1% of Patients During Clinical Trials
Digestive System—Gastritis, peptic ulcer with/without perforation, gastrointestinal hemorrhage, anorexia, flatulence, dry mouth, and blood in the stool. Increases in alkaline phosphatase, LDH, SGOT, jaundice, and cholestatic hepatitis, aphthous ulcerations of the buccal mucosa, metallic taste, and pancreatitis.
Cardiovascular—Atrial fibrillation, pulmonary edema, electrocardiographic changes, and supraventricular tachycardia.
Genitourinary Tract—Renal failure, dysuria, cystitis, hematuria, oliguria, azotemia, anuria, interstitial nephritis, nephrosis, and papillary necrosis.
Hypersensitivity—Angioedema (angioneurotic edema).
Hematologic—Purpura, bruising, hemorrhage, thrombocytopenia, hemolytic anemia, aplastic anemia, agranulocytosis, and pancytopenia.
Nervous System—Depression, disorientation, seizures, and trigeminal neuralgia.
Special Senses—Burning tongue, diplopia, and optic neuritis.
Skin and Appendages—Exfoliative dermatitis, toxic epidermal necrolysis, Stevens-Johnson syndrome, and alopecia.
Miscellaneous—Anaphylaxis, urticaria, malaise, insomnia, tachycardia, personality change, lymphadenopathy, mastodynia, and fever.
Most common adverse reactions (incidence ≥ 5%) are Dyspepsia, headache, somnolence, nausea, dizziness, constipation, nervousness, asthenia, and peripheral edema. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Xspire Pharma at 1-601-990-9497 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch (6)
USE IN SPECIFIC POPULATIONS SECTION
8. USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Use of NSAIDs, including NALFON, during the third trimester of pregnancy
increases the risk of premature closure of the fetal ductus arteriosus. Avoid
use of NSAIDs, including NALFON, in pregnant women starting at 30 weeks of
gestation (third trimester).
There are no adequate and well-controlled studies of NALFON in pregnant women. Data from observational studies regarding potential embryofetal risks of NSAID use in women in the first or second trimesters of pregnancy are inconclusive. In the general U.S. population, all clinically recognized pregnancies, regardless of drug exposure, have a background rate of 2-4% for major malformations, and 15-20% for pregnancy loss.
In animal reproduction studies, embryo-fetal lethality and skeletal abnormalities were noted in offspring of pregnant rabbits following oral administration of fenoprofen during organogenesis at 0.6 times the maximum human daily dose of 3200 mg/day. However, there were no major malformations noted following oral administration of fenoprofen calcium to pregnant rats and rabbits during organogenesis at exposures up to 0.3 and 0.6 times the maximum human daily dose of 3200 mg/day.
Based on animal data, prostaglandins have been shown to have an important role in endometrial vascular permeability, blastocyst implantation, and decidualization. In animal studies, administration of prostaglandin synthesis inhibitors such as fenoprofen, resulted in increased pre- and post- implantation loss.
Clinical Considerations
Labor or Delivery
There are no studies on the effects of NALFON during labor or delivery. In
animal studies, NSAIDS, including fenoprofen, inhibit prostaglandin synthesis,
cause delayed parturition, and increase the incidence of stillbirth.
Data
Human Data
There are no adequate and well-controlled studies of NALFON in pregnant women.
Data from observational studies regarding potential embryofetal risks of NSAID
use in women in the first or second trimesters of pregnancy are inconclusive.
Animal data
Pregnant rats were treated with fenoprofen using oral doses of 50 or 100 mg/kg
(0.15 times and 0.3 times the maximum human daily dose (MHDD) of 3200 mg/day
based on body surface area comparison) during the period of organogenesis. No
major malformations were noted and there was no evidence of maternal toxicity
at these doses, however, the exposures were below the exposures that will
occur in humans.
Pregnant rabbits were treated with fenoprofen using oral doses of 50 or 100
mg/kg (0.3 times and 0.6 times the MHDD of 3200 mg/day based on body surface
area comparison) during the period of organogenesis. Maternal toxicity
(mortality) was noted in the high dose animals. Although no major
malformations were noted, there was an increased incidence of embryo-fetal
lethality and skeletal abnormalities were present at 0.6 times the MHDD.
Pregnant rats were treated from Gestation Day 14 through Post-Natal Day 20
with oral doses of fenoprofen of 6.25, 12.5, 25, 50, or 100 mg/kg (0.02, 0.04,
0.08, 0.15, or 0.3 times the MDD of 3200 mg/day based on body surface area
comparison). All doses produced significant toxicity, including vaginal
bleeding, prolonged parturition, increased stillbirths, and maternal deaths.
Pregnant rats were treated from Gestation Day 6 through Gestation Day 19 and
Post Partum Day 1 to 20 (excluding parturition) with an oral dose of
fenoprofen of 100 mg/kg (0.3 times the MDD of 3200 mg/day based on body
surface area comparison) demonstrated only a small increase in the incidence
of impaired parturition despite the presence of maternal toxicity
(gastrointestinal ulceration and renal toxicity).
8.2 Lactation
Risk Summary
In a published study, after a dose of 600 mg every 6 hours for 4 days in
postpartum mothers, breastmilk fenoprofen levels were reportedly 1.6% of those
in maternal plasma. The developmental and health benefits of breastfeeding
should be considered along with the mother’s clinical need for NALFON and any
potential adverse effects on the breastfed infant from the NALFON or from the
underlying maternal condition.
8.3 Females and Males of Reproductive Potential
Infertility
Females
Based on the mechanism of action, the use of prostaglandin-mediated NSAIDs,
including NALFON, may delay or prevent rupture of ovarian follicles, which has
been associated with reversible infertility in some women. Published animal
studies have shown that administration of prostaglandin synthesis inhibitors
has the potential to disrupt prostaglandinmediated follicular rupture required
for ovulation. Small studies in women treated with NSAIDs have also shown a
reversible delay in ovulation. Consider withdrawal of NSAIDs, including
NALFON, in women who have difficulties conceiving or who are undergoing
investigation of infertility.
8.4 Pediatric Use
Safety and effectiveness in pediatric patients under the age of 18 have not been established.
8.5 Geriatric Use
Elderly patients, compared to younger patients, are at greater risk for NSAID- associated serious cardiovascular, gastrointestinal, and/or renal adverse reactions. If the anticipated benefit for the elderly patient outweighs these potential risks, start dosing at the low end of the dosing range, and monitor patients for adverse effects [ see Warnings and Precautions ( 5.1, 5.2, 5.3, 5.6, 5.13) ].
Pregnancy: Use of NSAIDs during the third trimester of pregnancy increases the risk of premature closure of the fetal ductus arteriosus. Avoid use of NSAIDs in pregnant women starting at 30 weeks gestation ( 5.10, 8.1)
Infertility: NSAIDs are associated with reversible infertility. Consider withdrawal of NALFON in women who have difficulties conceiving ( 8.3)
RECENT MAJOR CHANGES SECTION
RECENT MAJOR CHANGES
|
Boxed Warning |
4/2016 |
|
Warnings and Precautions, Cardiovascular Thrombotic Events ( 5.1) |
4/2016 |
|
Warnings and Precautions, Heart Failure and Edema ( 5.5) |
4/2016 |
DOSAGE & ADMINISTRATION SECTION
2. DOSAGE AND ADMINISTRATION
2.1 General Dosing Instructions
Carefully consider the potential benefits and risks of NALFON and other
treatment options before deciding to use NALFON. Use lowest effective dosage
for the shortest duration consistent with individual patient treatment goals [ see Warnings and Precautions ( 5) ].
Nalfon may be administered with meals or with milk. Although the total amount
absorbed is not affected, peak blood levels are delayed and diminished.
Patients with rheumatoid arthritis generally seem to require larger doses of
Nalfon than do those with osteoarthritis. The smallest dose that yields
acceptable control should be employed.
Although improvement may be seen in a few days in many patients, an additional
2 to 3 weeks may be required to gauge the full benefits of therapy.
2.2 Analgesia
For the treatment of mild to moderate pain, the recommended dosage is 200 mg given orally every 4 to 6 hours, as needed.
2.3 Rheumatoid Arthritis and Osteoarthritis
For the relief of signs and symptoms of rheumatoid arthritis or osteoarthritis the recommended dose is 400 to 600 mg given orally, 3 or 4 times a day. The dose should be tailored to the needs of the patient and may be increased or decreased depending on the severity of the symptoms. Dosage adjustments may be made after initiation of drug therapy or during exacerbations of the disease. Total daily dosage should not exceed 3,200 mg.
•
Use the lowest effective dosage for shortest duration consistent with individual patient treatment goals ( 2.1)
•
Analgesia: For the treatment of mild to moderate pain, the recommended dosage is 200 mg given orally every 4 to 6 hours, as needed ( 2.1)
•
Rheumatoid Arthritis and Osteoarthritis: For the relief of signs and symptoms of rheumatoid arthritis or osteoarthritis the recommended dose is 400 to 600 mg given orally, 3 or 4 times a day. The dose should be tailored to the needs of the patient and may be increased or decreased depending on the severity of the symptoms. Dosage adjustments may be made after initiation of drug therapy or during exacerbations of the disease. Total daily dosage should not exceed 3,200 mg.
DOSAGE FORMS & STRENGTHS SECTION
3. DOSAGE FORMS AND STRENGTHS
Nalfon ® (fenoprofen calcium) capsules:
•
The 200 mg capsule is opaque yellow No. 97 cap and opaque white body, imprinted with "RX681" on the cap and body.
•
The 400 mg capsule is opaque green cap and opaque blue body, imprinted with "NALFON 400 mg" on the cap and "EP 123" on the body.
NALFON (fenoprofen calcium) capsules: 200 mg and 400 mg ( 3)
OVERDOSAGE SECTION
10. OVERDOSAGE
Symptoms following acute NSAID overdosages have been typically limited to lethargy, drowsiness, nausea, vomiting, and epigastric pain, which have been generally reversible with supportive care. Gastrointestinal bleeding has occurred. Hypertension, acute renal failure, respiratory depression, and coma have occurred, but were rare [ see Warnings and Precautions ( 5.1, 5.2, 5.4, 5.6) ].
Manage patients with symptomatic and supportive care following an NSAID overdosage. There are no specific antidotes. Consider emesis and/or activated charcoal (60 to 100 grams in adults, 1 to 2 grams per kg of body weight in pediatric patients) and/or osmotic cathartic in symptomatic patients seen within four hours of ingestion or in patients with a large overdosage (5 to 10 times the recommended dosage). Forced diuresis, alkalinization of urine, hemodialysis, or hemoperfusion may not be useful due to high protein binding.
For additional information about overdosage treatment contact a poison control center (1-800-222-1222).
DESCRIPTION SECTION
11. DESCRIPTION
NALFON (fenoprofen calcium, USP) capsules is a nonsteroidal, anti-inflammatory drug available in 200 mg and 400 mg capsule form for oral administration.
The 200 mg capsule is opaque yellow No. 97 cap and opaque white body, imprinted with “RX681” on the cap and body.
The 400 mg capsule is opaque green cap and opaque blue body, imprinted with “NALFON 400 mg" on the cap and “EP 123” on the body.
The chemical name is Benzenaecetic acid, α-methyl-3-phenoxy-, calcium salt dihydrate, (±)-. The molecular weight is 558.65. Its molecular formula is C 30H 26CaO 6•2H 2O, and it has the following chemical structure.
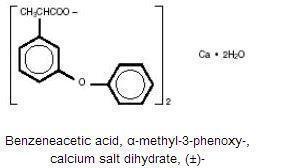
Fenoprofen Calcium is an arylacetic acid derivative. It is a white crystalline powder. At 25°C, it dissolves to a 15 mg/mL solution in alcohol (95%). It is slightly soluble in water and insoluble in benzene.The pKa of fenoprofen calcium is 4.5 at 25°C.
Nalfon capsules contain fenoprofen calcium as the dihydrate in an amount equivalent to 200 mg (0.826 mmol) or 400 mg (1.65 mmol) of fenoprofen.
Inactive ingredients in Nalfon capsules are crospovidone, magnesium stearate, sodium lauryl sulfate, and talc. In addition, the 200 mg capsules contain gelatin, titanium dioxide, yellow iron oxide, and red iron oxide, and the 400 mg capsules contain gelatin, D&C Yellow #10, FD&C Blue #1, FD&C Red #40, FD&C Yellow #6, and titanium dioxide.
CLINICAL PHARMACOLOGY SECTION
12. CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Fernoprofen has analgesic, anti-inflammatory, and antipyretic properties.
The mechanism of action of NALFON, like that of other NSAIDs, is not completely understood but involves inhibition of cyclooxygenase (COX-1 and COX-2).
Fenoprofen is a potent inhibitor of prostaglandin synthesis in vitro. Fenoprofen concentrations reached during therapy have produced in vivo effects. Prostaglandins sensitize afferent nerves and potentiate the action of bradykinin in inducing pain in animal models. Prostaglandins are mediators of inflammation. Because fenoprofen is an inhibitor of prostaglandin synthesis, its mode of action may be due to a decrease of prostaglandins in peripheral tissues.
12.3 Pharmacokinetics
Absorption
Under fasting conditions, fenoprofen is rapidly absorbed, and peak plasma
levels of 50 mcg/L are achieved within 2 hours after oral administration of
600 mg doses. Good dose proportionality was observed between 200 and 600 mg
doses in fasting male volunteers.
Distribution
Fenoprofen is highly bound (99%) to albumin.
Elimination
Metabolism
The plasma half-life is approximately 3 hours.
Excretion
About 90% of a single oral dose is eliminated within 24 hours as fenoprofen
glucuronide and 4'-hydroxyfenoprofen glucuronide, the major urinary
metabolites of fenoprofen.
Specific Populations
Gertatrics
Peak plasma levels of fenoprofen in normal elderly volunteers were similar to
those observed in normal young volunteers. Elderly volunteers had a mean
plasma clearance of 2.2 L/hour while plasma clearance of fenoprofen in normal
young volunteers ranged from 3 to 3.5 L/hour. The overall elimination rate
constant, plasma half-life and ratio of renal to nonrenal clearance of
fenoprofen was the same in elderly and young volunteers. The 30 to 60%
decrease in plasma clearance is due to a decrease in the volume of
distribution in the body.
Drug Interaction Studies
Aspirin: When NSAIDs were administered with aspirin, the protein binding of
NSAIDs were reduced, although the clearance of free NSAID was not altered. The
clinical significance of this interaction is not known. See Table 1 for
clinically significant drug interactions of NSAIDs with aspirin [ see Drug Interactions ( 7) ].
Antacid: The concomitant administration of antacid (containing both aluminum and magnesium hydroxide) does not interfere with absorption of fenoprofen.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility
Carcinogenesis
Long-term studies in animals to evaluate the carcinogenic potential of
fenoprofen have not been conducted.
Mutagenesis
Studies to evaluate the genotoxic potential of fenoprofen have not been
conducted.
Impairment of Fertility
Female and Male rats were treated with 60 to 70 mg/kg/day or 120 to 150
mg/kg/day fenoprofen calcium via the diet (approximately 0.2 or 0.4 times the
maximum human daily dose of 3200 mg/day based on body surface area comparison,
respectively). Male rats were treated from 77 days prior to mating and during
mating. Female rats were treated from 14 days prior to mating and through
gestation. Pregnancy rates were slightly reduced in the low and high dose
groups compared to controls. There was no adverse effect on implantations,
resorptions, or live fetuses.
CLINICAL STUDIES SECTION
14. CLINICAL STUDIES
NALFON is a nonsteroidal, anti-inflammatory, antiarthritic drug that also possesses analgesic and antipyretic activities. Its exact mode of action is unknown, but it is thought that prostaglandin synthetase inhibition is involved.
Results in humans demonstrate that fenoprofen has both anti-inflammatory and analgesic actions. The emergence and degree of erythemic response were measured in adult male volunteers exposed to ultraviolet irradiation. The effects of NALFON, aspirin, and indomethacin were each compared with those of a placebo. All 3 drugs demonstrated antierythemic activity.
In all patients with rheumatoid arthritis, the anti-inflammatory action of NALFON has been evidenced by relief of pain, increase in grip strength, and reductions in joint swelling, duration of morning stiffness, and disease activity (as assessed by both the investigator and the patient). The anti- inflammatory action of NALFON has also been evidenced by increased mobility (i.e., a decrease in the number of joints having limited motion).
The use of NALFON in combination with gold salts or corticosteroids has been studied in patients with rheumatoid arthritis. The studies, however, were inadequate in demonstrating whether further improvement is obtained by adding NALFON to maintenance therapy with gold salts or steroids. Whether or not NALFON used in conjunction with partially effective doses of a corticosteroid has a “steroid-sparing” effect is unknown.
In patients with osteoarthritis, the anti-inflammatory and analgesic effects of NALFON have been demonstrated by reduction in tenderness as a response to pressure and reductions in night pain, stiffness, swelling, and overall disease activity (as assessed by both the patient and the investigator). These effects have also been demonstrated by relief of pain with motion and at rest and increased range of motion in involved joints.
In patients with rheumatoid arthritis and osteoarthritis, clinical studies have shown NALFON to be comparable to aspirin in controlling the aforementioned measures of disease activity, but mild gastrointestinal reactions (nausea, dyspepsia) and tinnitus occurred less frequently in patients treated with NALFON than in aspirin-treated patients. It is not known whether NALFON causes less peptic ulceration than does aspirin.
In patients with pain, the analgesic action of Nalfon has produced a reduction in pain intensity, an increase in pain relief, improvement in total analgesia scores, and a sustained analgesic effect.
HOW SUPPLIED SECTION
16. HOW SUPPLIED/STORAGE AND HANDLING
Nalfon ® (fenoprofen calcium, USP) are available in capsule form for oral administration, and are supplied as following:
•
The 400 mg capsule has an opaque green cap and an opaque blue body, imprinted with "NALFON 400 mg" on the cap and "EP 123" on the body.
NDC 63187-706-30 Bottles of 30.
NDC 63187-706-60 Bottles of 60
NDC 63187-706-90 Bottles of 90
Storage:
Store at room temperature 20°C to 25°C (68°F to 77°F); excursions permitted
between 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
Preserve in well-closed containers.
INFORMATION FOR PATIENTS SECTION
17. PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide) that accompanies each prescription dispensed. Inform patients, families, or their caregivers of the following information before initiating therapy with NALFON and periodically during the course of ongoing therapy.
Cardiovascular Thrombotic EventsAdvise patients to be alert for the symptoms of cardiovascular thrombotic events, including chest pain, shortness of breath, weakness, or slurring of speech, and to report any of these symptoms to their health care provider immediately [ see Warnings and Precautions ( 5.1) ].
Gastrointestinal Bleeding, Ulceration, and Perforation
Advise patients to report symptoms of ulcerations and bleeding, including
epigastric pain, dyspepsia, melena, and hematemesis to their health care
provider. In the setting of concomitant use of low-dose aspirin for cardiac
prophylaxis, inform patients of the increased risk for and the signs and
symptoms of GI bleeding [ see Warnings and Precautions ( 5.2) ].
Hepatotoxicity
Inform patients of the warning signs and symptoms of hepatotoxicity (e.g.,
nausea, fatigue, lethargy, pruritus, diarrhea, jaundice, right upper quadrant
tenderness, and “flu-like” symptoms). If these occur, instruct patients to
stop NALFON and seek immediate medical therapy [ see Warnings and Precautions ( 5.3) ].
Heart Failure and Edema
Advise patients to be alert for the symptoms of congestive heart failure
including shortness of breath, unexplained weight gain, or edema and to
contact their healthcare provider if such symptoms occur [ see Warnings and Precautions ( 5.5) ].
Anaphylactic Reactions
Inform patients of the signs of an anaphylactic reaction (e.g., difficulty
breathing, swelling of the face or throat). Instruct patients to seek
immediate emergency help if these occur [ see Contraindications ( 4) and Warnings and Precautions ( 5.7) ].
Serious Skin Reactions
Advise patients to stop NALFON immediately if they develop any type of rash
and to contact their healthcare provider as soon as possible [ see Warnings and Precautions ( 5.9) ].
Female Fertility
Advise females of reproductive potential who desire pregnancy that NSAIDs,
including NALFON, may be associated with a reversible delay in ovulation [ see Use in Specific Populations ( 8.3) ]
Fetal Toxicity
Inform pregnant women to avoid use of NALFON and other NSAIDs starting at 30
weeks gestation because of the risk of the premature closing of the fetal
ductus arteriosus [ see Warnings and Precautions ( 5.10) and Use in Specific Populations ( 8.1) ].
Avoid Concomitant Use of NSAIDs
Inform patients that the concomitant use of NALFON with other NSAIDs or
salicylates (e.g., diflunisal, salsalate) is not recommended due to the
increased risk of gastrointestinal toxicity, and little or no increase in
efficacy [ see Warnings and Precautions ( 5.2) and Drug Interactions ( 7) ].
Alert patients that NSAIDs may be present in “over the counter” medications
for treatment of colds, fever, or insomnia.
Use of NSAIDS and Low-Dose Aspirin
Inform patients not to use low-dose aspirin concomitantly with NALFON until
they talk to their healthcare provider [ see Drug Interactions ( 7) ].
Manufactured for:
Xspire Pharma
Ridgeland, MS. 39157
Issued: 04/ 2016
SPL MEDGUIDE SECTION
Medication Guide for Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
What is the most important information I should know about medicines called Nonsteroidal Antiinflammatory Drugs (NSAIDs)?
NSAIDs can cause serious side effects, including:
•
**Increased risk of a heart attack or stroke that can lead to death.**This risk may happen early in treatment and may increase:
•
with increasing doses of NSAIDs
•
with longer use of NSAIDs
Do not take NSAIDs right before or after a heart surgery called a “coronary artery bypass graft (CABG)."
Avoid taking NSAIDs after a recent heart attack, unless your healthcare provider tells you to. You may have an increased risk of another heart attack if you take NSAIDs after a recent heart attack
•
**Increased risk of bleeding, ulcers, and tears (perforation) of the esophagus (tube leading from the mouth to the stomach), stomach and intestines:**
•
anytime during use
•
without warning symptoms
•
that may cause death
The risk of getting an ulcer or bleeding increases with:
NSAIDs should only be used:
What are NSAIDs?
NSAIDs are used to treat pain and redness, swelling, and heat (inflammation) from medical conditions such as different types of arthritis, menstrual cramps, and other types of short-term pain.
Who should not take NSAIDs?
Do not take NSAIDs:
•
if you have had an asthma attack, hives, or other allergic reaction with aspirin or any other NSAIDs
•
right before or after heart bypass surgery.
Before taking NSAIDS, tell your healthcare provider about all of your medical conditions, including if you:
•
have liver or kidney problems
•
have high blood pressure
•
have asthma
•
are pregnant or plan to become pregnant. Talk to your healthcare provivder if you are considering taking NSAIDs during pregnancy.**You should not take NSAIDs after 29 weeks of pregnancy.**
•
are breastfeeding or plan to breast feed.
Tell your healthcare provider about all of the medicines you take, including prescription or over-the-counter medicines, vitamins or herbal supplements. NSAIDs and some other medicines can interact with each other and cause serious side effects.Do not start taking any new medicine without talking to your healthcare provider first.
What are the possible side effects of NSAIDs?
NSAIDs can cause serious side effects, including:
See “What is the most important information I should know about medicines called Nonsteroidal Anti-inflammatory Drugs (NSAIDs)?"
•
new or worse high blood pressure
•
heart failure
•
liver problems including liver failure
•
kidney problems including kidney failure
•
low red blood cells (anemia)
•
life-threatening skin reactions
•
life-threatening allergic reactions
•
**Other side effects of NSAIDs include:**stomach pain, constipation, diarrhea, gas, heartburn, nausea, vomiting, and dizziness
Get emergency help right away if you get any of the following symptoms:
•
shortness of breath or trouble breathing
•
chest pain
•
weakness in one part or side of your body
•
slurred speech
•
swelling of the face or throat
Stop taking your NSAID and call your healthcare provider right away if you get any of the following symptoms:
•
nausea
•
more tired or weaker than usual
•
diarrhea
•
itching
•
your skin or eyes look yellow
•
indigestion or stomach pain
•
flu-like symptoms
•
vomit blood
•
there is blood in your bowel movement or it is black and sticky like tar
•
unusual weight gain
•
skin rash or blisters with fever
•
swelling of the arms, legs, hands and feet
**If you take too much of your NSAID, call your healthcare provider or get medical help right away.**These are not all the possible side effects of NSAIDs. For more information, ask your healthcare provider or pharmacist about NSAIDs.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Other information about NSAIDs
•
Aspirin is an NSAID but it does not increase the chance of a heart attack. Aspirin can cause bleeding in the brain, stomach, and intestines. Aspirin can also cause ulcers in the stomach and intestines.
•
Some NSAIDs are sold in lower doses without a prescription (over-the counter). Talk to your healthcare provider before using over-the-counter NSAIDs for more than 10 days.
General information about the safe and effective use of NSAIDs
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use NSAIDs for a condition for which it was not prescribed. Do not give NSAIDs to other people, even if they have the same symptoms that you have. It may harm them.
If you would like more information about NSAIDs, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about NSAIDs that is written for health professionals.
Manufactured for:
Xspire Pharma
Ridgeland, MS. 39157
For more information, go to www.nalfon.com or call 1-601-990-9497.
This Medication Guide has been approved by the U.S. Food and Drug
Administration.
Issued: 04/2016
