Thiothixene
Thiothixene
9480a406-c85b-ba06-e053-2a95a90a0912
HUMAN PRESCRIPTION DRUG LABEL
Aug 24, 2023
Golden State Medical Supply, Inc.
DUNS: 603184490
Products 4
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Thiothixene
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (19)
Thiothixene
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (18)
Thiothixene
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (19)
Thiothixene
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (18)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Thiothixene Capsules, USP 1 mg 100's counts

Thiothixene Capsules, USP 2 mg 100'scounts

Thiothixene Capsules, USP 5 mg 100'scounts

Thiothixene Capsules, USP 10 mg 100'scounts

DESCRIPTION SECTION
DESCRIPTION
Thiothixene is a thioxanthene derivative. Specifically, it is the cis isomer of N,N-dimethyl-9-[3-(4- methyl-1-piperazinyl)propylidene]thioxanthene-2-sulfonamide. It may be represented by the following structural formula
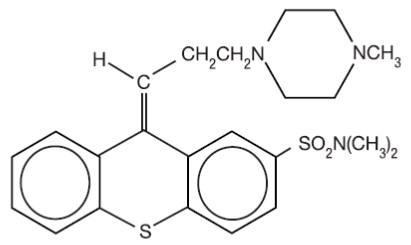
The thioxanthenes differ from the phenothiazines by the replacement of nitrogen in the central ring with a carbon-linked side chain fixed in space in a rigid structural configuration. An N,N-dimethyl sulfonamide functional group is bonded to the thioxanthene nucleus.
Each capsule contains 1 mg, 2 mg, 5 mg or 10 mg of thiothixene, USP and the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, pregelatinized starch and sodium lauryl sulfate. Each of the empty gelatin capsules contains FD&C Blue No. 1, FD&C Red No. 40, FD&C Yellow No. 6, gelatin, sodium lauryl sulfate and titanium dioxide. In addition, the 1 mg empty gelatin capsules contain D&C Red No. 28 and the 2 mg empty gelatin capsules contain D&C Yellow No. 10.
The imprinting ink contains the following: ammonia solution, black iron oxide, potassium hydroxide, propylene glycol and shellac.
