Epinephrine
These highlights do not include all the information needed to use EPINEPHRINE INJECTION, 0.3 mg and EPINEPHRINE INJECTION, 0.15 mg safely and effectively. See full prescribing information for EPINEPHRINE INJECTION, 0.3 mg and EPINEPHRINE INJECTION, 0.15 mg. EPINEPHRINE injection, 0.3 mg, for intramuscular or subcutaneous use EPINEPHRINE injection, 0.15 mg, for intramuscular or subcutaneous use Initial U.S. Approval: 1939
04f07f15-55e2-411b-abb8-07eab37f5664
HUMAN PRESCRIPTION DRUG LABEL
Jan 31, 2021
Teva Pharmaceuticals USA, Inc.
DUNS: 001627975
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Epinephrine
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Epinephrine
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Package/Label Display Panel
NDC 0093-5985-27
2-Pack
Epinephrine Injection USP, 0.15 mg (Single-Dose Auto-Injectors)
For Allergic Emergencies (Anaphylaxis)
Each carton contains:
Two green Epinephrine Injection USP, 0.15 mg
(Single-Dose Auto-Injectors)
One grey Trainer
Rx only
2 Single Dose Auto-Injectors and 1 Trainer
TEVA

INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
Epinephrine Injection, 0.3 mg and Epinephrine Injection, 0.15 mg are indicated in the emergency treatment of allergic reactions (Type I) including anaphylaxis to stinging insects (e.g., order Hymenoptera, which include bees, wasps, hornets, yellow jackets and fire ants) and biting insects (e.g., triatoma, mosquitoes), allergen immunotherapy, foods, drugs, diagnostic testing substances (e.g., radiocontrast media) and other allergens, as well as idiopathic anaphylaxis or exercise-induced anaphylaxis.
Epinephrine Injection, 0.3 mg and Epinephrine Injection, 0.15 mg are intended for immediate administration in patients who are determined to be at increased risk for anaphylaxis, including individuals with a history of anaphylactic reactions.
Anaphylactic reactions may occur within minutes after exposure and consist of flushing, apprehension, syncope, tachycardia, thready or unobtainable pulse associated with a fall in blood pressure, convulsions, vomiting, diarrhea and abdominal cramps, involuntary voiding, wheezing, dyspnea due to laryngeal spasm, pruritus, rashes, urticaria or angioedema.
Epinephrine Injection, 0.3 mg and Epinephrine Injection, 0.15 mg are intended for immediate administration as emergency supportive therapy only and are not a substitute for immediate medical care.
Epinephrine Injection, 0.3 mg and Epinephrine Injection, 0.15 mg contain epinephrine, are non-selective alpha and beta-adrenergic receptor agonist indicated in the emergency treatment of allergic reactions (Type I) including anaphylaxis. (1)
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
None
None (4)
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Emergency Treatment
Epinephrine injection, 0.3 mg and epinephrine injection, 0.15 mg are intended for immediate administration as emergency supportive therapy and are not intended as a substitute for immediate medical care.**In conjunction with the administration of epinephrine, the patient should seek immediate medical or hospital care.**More than two sequential doses of epinephrine should only be administered under direct medical supervision [see Indications and Usage (1), Dosage and Administration (2) and Patient Counseling Information (17)].
5.2 Injection-Related Complications
Epinephrine injection, 0.3 mg and epinephrine injection, 0.15 mg should**only **be injected into the anterolateral aspect of the thigh [see Dosage and Administration (2) and Patient Counseling Information (17)].
Do not inject intravenously
Large doses or accidental intravenous injection of epinephrine may result in
cerebral hemorrhage due to sharp rise in blood pressure. Rapidly acting
vasodilators can counteract the marked pressor effects of epinephrine if there
is such inadvertent administration.
Do not inject into buttock
****Injection into the buttock may not provide effective treatment of
anaphylaxis. Advise the patient to go immediately to the nearest emergency
room for further treatment of anaphylaxis. Additionally, injection into the
buttock has been associated with Clostridial infections (gas gangrene).
Cleansing with alcohol does not kill bacterial spores, and therefore, does not
lower this risk.
Do not inject into digits, hands or feet
****Since epinephrine is a strong vasoconstrictor, accidental injection into
the digits, hands or feet may result in loss of blood flow to the affected
area. Advise the patient to go immediately to the nearest emergency room and
to inform the healthcare provider in the emergency room of the location of the
accidental injection. Treatment of such inadvertent administration should
consist of vasodilation, in addition to further appropriate treatment of
anaphylaxis [see Adverse Reactions (6)].
****Hold leg firmly during injection
Lacerations, bent needles, and embedded needles have been reported when
epinephrine injection, 0.3 mg and epinephrine injection, 0.15 mg have been
injected into the thigh of young children who are uncooperative and kick or
move during an injection. To minimize the risk of injection related injury
when administering, hold the child’s leg firmly in place and limit movement
prior to and during injection.
5.3 Serious Infections at the Injection Site
Rare cases of serious skin and soft tissue infections, including necrotizing fasciitis and myonecrosis caused by Clostridia (gas gangrene), have been reported at the injection site following epinephrine injection for anaphylaxis. Clostridium spores can be present on the skin and introduced into the deep tissue with subcutaneous or intramuscular injection. While cleansing with alcohol may reduce presence of bacteria on the skin, alcohol cleansing does not kill Clostridium spores. To decrease the risk of Clostridium infection, do not inject epinephrine injection into the buttock [see Warnings and Precautions (5.2)]. Advise patients to seek medical care if they develop signs or symptoms of infection, such as persistent redness, warmth, swelling, or tenderness, at the epinephrine injection site.
5.4 Allergic Reactions Associated with Sulfite
The presence of a sulfite in this product should not deter administration of the drug for treatment of serious allergic or other emergency situations even if the patient is sulfite-sensitive.
Epinephrine is the preferred treatment for serious allergic reactions or other emergency situations even though this product contains sodium metabisulfite, a sulfite that may, in other products, cause allergic-type reactions including anaphylactic symptoms or life-threatening or less severe asthmatic episodes in certain susceptible persons.
The alternatives to using epinephrine in a life-threatening situation may not be satisfactory.
5.5 Disease Interactions
Some patients may be at greater risk for developing adverse reactions after epinephrine administration. Despite these concerns, it should be recognized that the presence of these conditions is not a contraindication to epinephrine administration in an acute, life-threatening situation. Therefore, patients with these conditions, and/or any other person who might be in a position to administer epinephrine injection, 0.3 mg or epinephrine injection, 0.15 mg to a patient experiencing anaphylaxis should be carefully instructed in regard to the circumstances under which epinephrine should be used.
Patients with Heart Disease
Epinephrine should be administered with caution to patients who have heart disease, including patients with cardiac arrhythmias, coronary artery or organic heart disease, or hypertension. In such patients, or in patients who are on drugs that may sensitize the heart to arrhythmias, epinephrine may precipitate or aggravate angina pectoris as well as produce ventricular arrhythmias [see Drug Interactions (7) and Adverse Reactions (6)].
Other Patients and Diseases
Epinephrine should be administered with caution to patients with hyperthyroidism, diabetes, elderly individuals, and pregnant women. Patients with Parkinson’s disease may notice a temporary worsening of symptoms.
- In conjunction with use, seek immediate medical or hospital care. (5.1)
- Do not inject intravenously, into buttock, or into digits, hands, or feet. (5.2)
- To minimize the risk of injection related injury, hold the child’s leg firmly in place and limit movement prior to and during injection when administering to young children. (5.2)
- Rare cases of serious skin and soft tissue infections have been reported following epinephrine injection. Advise patients to seek medical care if they develop signs or symptoms of infection. (5.3)
- The presence of a sulfite in this product should not deter use. (5.4)
- Administer with caution in patients with heart disease; may aggravate angina pectoris or produce ventricular arrhythmias. (5.5)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
Due to the lack of randomized, controlled clinical trials of epinephrine for the treatment of anaphylaxis, the true incidence of adverse reactions associated with the systemic use of epinephrine is difficult to determine. Adverse reactions reported in observational trials, case reports, and studies are listed below.
Common adverse reactions to systemically administered epinephrine include anxiety; apprehensiveness; restlessness; tremor; weakness; dizziness; sweating; palpitations; pallor; nausea and vomiting; headache; and/or respiratory difficulties. These symptoms occur in some persons receiving therapeutic doses of epinephrine, but are more likely to occur in patients with hypertension or hyperthyroidism [see Warnings and Precautions (5.5)].
Cardiovascular Reactions
- Arrhythmias, including fatal ventricular fibrillation, have been reported, particularly in patients with underlying cardiac disease or those receiving certain drugs [see Warnings and Precautions (5.5) and Drug Interactions (7)].
- Rapid rises in blood pressure have produced cerebral hemorrhage, particularly in elderly patients with cardiovascular disease [see Warnings and Precautions (5.5)].
- Angina may occur in patients with coronary artery disease [see Warnings and Precautions (5.5)].
- Rare cases of stress cardiomyopathy have been reported in patients treated with epinephrine.
Reactions from Accidental Injection and/or Improper Technique
- Accidental injection into the digits, hands or feet may result in loss of blood flow to the affected area [see Warnings and Precautions (5.2)].
- Adverse reactions experienced as a result of accidental injections may include increased heart rate, local reactions including injection site pallor, coldness and hypoesthesia or injury at the injection site resulting in bruising, bleeding, discoloration, erythema or skeletal injury.
- Lacerations, bent needles, and embedded needles have been reported when epinephrine injection has been injected into the thigh of young children who are uncooperative and kick or move during the injection [see Warnings and Precautions (5.2)].
- Injection into the buttock has resulted in cases of gas gangrene [see Warnings and Precautions (5.2)].
Skin and Soft Tissue Infections
- Rare cases of serious skin and soft tissue infections, including necrotizing fasciitis and myonecrosis caused by Clostridia (gas gangrene), have been reported following epinephrine injection, including epinephrine injection 0.3 mg, in the thigh [see Warnings and Precautions (5.3)].
Adverse reactions to epinephrine include anxiety, apprehensiveness, restlessness, tremor, weakness, dizziness, sweating, palpitations, pallor, nausea and vomiting, headache, and/or respiratory difficulties. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Teva Pharmaceuticals USA, Inc. at 1-888-838-2872 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
Cardiac Glycosides, Diuretics, and Anti-arrhythmics
Patients who receive epinephrine while concomitantly taking cardiac glycosides, diuretics, or anti-arrhythmics should be observed carefully for the development of cardiac arrhythmias [see Warnings and Precautions (5.5)].
Antidepressants, Monoamine Oxidase Inhibitors, Levothyroxine, and Antihistamines
The effects of epinephrine may be potentiated by tricyclic antidepressants, monoamine oxidase inhibitors, levothyroxine sodium, and certain antihistamines, notably chlorpheniramine, tripelennamine, and diphenhydramine.
Beta-Adrenergic Blockers
The cardiostimulating and bronchodilating effects of epinephrine are antagonized by beta-adrenergic blocking drugs, such as propranolol.
Alpha-Adrenergic Blockers
The vasoconstricting and hypertensive effects of epinephrine are antagonized by alpha-adrenergic blocking drugs, such as phentolamine.
Ergot Alkaloids
Ergot alkaloids may also reverse the pressor effects of epinephrine.
- Cardiac glycosides or diuretics: observe for development of cardiac arrhythmias. (7)
- Tricyclic antidepressants, monoamine oxidase inhibitors, levothyroxine sodium, and certain antihistamines potentiate effects of epinephrine. (7)
- Beta-adrenergic blocking drugs antagonize cardiostimulating and bronchodilating effects of epinephrine. (7)
- Alpha-adrenergic blocking drugs antagonize vasoconstricting and hypertensive effects of epinephrine. (7)
- Ergot alkaloids may reverse the pressor effects of epinephrine. (7)
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage According to Patient Body Weight
- Patients greater than or equal to 30 kg (approximately 66 pounds or more): Epinephrine injection, 0.3 mg
- Patients 15 kg to 30 kg (33 pounds to 66 pounds): Epinephrine injection, 0.15 mg
2.2 Administration Instructions
- Inject the single-dose epinephrine injection, 0.3 mg or epinephrine injection, 0.15 mg intramuscularly or subcutaneously into the anterolateral aspect of the thigh, through clothing if necessary. Do not inject intravenously, and do not inject into buttocks, into digits, hands or feet [see Warnings and Precautions (5.2)].
- Instruct caregivers of young children who are prescribed an epinephrine injection, 0.3 mg or epinephrine injection, 0.15 mg and who may be uncooperative and kick or move during an injection to hold the leg firmly in place and limit movement prior to and during an injection [see Warnings and Precautions (5.2)].
- Each epinephrine injection, 0.3 mg or epinephrine injection, 0.15 mg is a single-dose epinephrine injection for single use. Since the doses of epinephrine delivered from epinephrine injection, 0.3 mg or epinephrine injection, 0.15 mg are fixed, consider using other forms of injectable epinephrine if doses lower than 0.15 mg are deemed necessary.
- With severe persistent anaphylaxis, repeat injections with an additional epinephrine injection, 0.3 mg or epinephrine injection, 0.15 mg may be necessary. More than two sequential doses of epinephrine should only be administered under direct medical supervision [see Warnings and Precautions (5.1)].
- The epinephrine solution in the clear window of the Epinephrine Injection 0.3 mg (Auto-Injector) or Epinephrine Injection 0.15 mg (Auto-Injector) should be inspected visually for particulate matter and discoloration.
Discarding After Use:
The epinephrine injection 0.3 mg and epinephrine injection 0.15 mg each contain 1 mL epinephrine solution. Approximately 0.7 mL remains in the auto- injector after activation, but is not available for future use, and should be discarded.
- Patients greater than or equal to 30 kg (66 lbs): Epinephrine injection, 0.3 mg (2)
- Patients 15 to 30 kg (33 lbs to 66 lbs): Epinephrine injection, 0.15 mg (2)
Inject intramuscularly or subcutaneously into the anterolateral aspect of the thigh, through clothing if necessary. Each device is a single-dose injection. (2)
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
- Injection: 0.3 mg (0.3 mg/0.3 mL), clear and colorless solution in single-dose pre-filled auto-injector
- Injection: 0.15 mg (0.15 mg/0.3 mL), clear and colorless solution in single-dose pre-filled auto-injector
- Injection: 0.3 mg (0.3 mg/0.3 mL) single-dose pre-filled auto-injector (3)
- Injection: 0.15 mg (0.15 mg/0.3 mL) single-dose pre-filled auto-injector (3)
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate and well controlled studies of the acute effect of epinephrine in pregnant women. In animal reproductive studies, epinephrine administered by the subcutaneous route to rabbits, mice, and hamsters during the period of organogenesis was teratogenic at doses 7 times and higher than the maximum recommended human intramuscular and subcutaneous dose on a mg/m2 basis. Epinephrine is the first-line medication of choice for the treatment of anaphylaxis during pregnancy in humans. Epinephrine should be used for treatment of anaphylaxis during pregnancy in the same manner as it is used in non-pregnant patients.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-associated maternal and embryo/fetal risk:
During pregnancy, anaphylaxis can be catastrophic and can lead to hypoxic-
ischemic encephalopathy and permanent central nervous system damage or death
in the mother and, more commonly, in the fetus or neonate. The prevalence of
anaphylaxis occurring during pregnancy is reported to be approximately 3 cases
per
100,000 deliveries.
Management of anaphylaxis during pregnancy is similar to management in the general population. Epinephrine is the first line-medication of choice for treatment of anaphylaxis; it should be used in the same manner in pregnant and non-pregnant patients. In conjunction with the administration of epinephrine, the patient should seek immediate medical or hospital care.
Data
Animal Data:
In an embryofetal development study with rabbits dosed during the period of organogenesis, epinephrine was shown to be teratogenic (including gastroschisis and embryonic lethality) at doses approximately 40 times the maximum recommended intramuscular or subcutaneous dose (on a mg/m2 basis at a maternal subcutaneous dose of 1.2 mg/kg/day for two to three days).
In an embryofetal development study with mice dosed during the period of organogenesis, epinephrine was shown to be teratogenic (including embryonic lethality) at doses approximately 8 times the maximum recommended intramuscular or subcutaneous dose (on a mg/m2 basis at maternal subcutaneous dose of 1 mg/kg/day for 10 days). These effects were not seen in mice at approximately 4 times the maximum recommended daily intramuscular or subcutaneous dose (on a mg/m2 basis at a subcutaneous maternal dose of 0.5 mg/kg/day for 10 days).
In an embryofetal development study with hamsters dosed during the period of organogenesis from gestation days 7 to 10, epinephrine was shown to be teratogenic at doses approximately 7 times the maximum recommended intramuscular or subcutaneous dose (on a mg/m2 basis at a maternal subcutaneous dose of 0.5 mg/kg/day).
8.2 Lactation
Risk Summary
There is no information on the presence of epinephrine in human milk, the effects on breastfed infants, or the effects on milk production. Epinephrine is the first line-medication of choice for treatment of anaphylaxis; it should be used in the same manner in breastfeeding and non-breastfeeding patients.
8.4 Pediatric Use
Epinephrine injection, 0.3 mg or epinephrine injection, 0.15 mg may be administered to pediatric patients at a dosage appropriate to body weight [see Dosage and Administration (2.1)]. Clinical experience with the use of epinephrine suggests that the adverse reactions seen in children are similar in nature and extent to those both expected and reported in adults. Since the doses of epinephrine delivered from epinephrine injection, 0.3 mg and epinephrine injection, 0.15 mg are fixed, consider using other forms of injectable epinephrine if doses lower than 0.15 mg are deemed necessary.
8.5 Geriatric Use
Clinical studies for the treatment of anaphylaxis have not been performed in subjects aged 65 and over to determine whether they respond differently from younger subjects. However, other reported clinical experience with use of epinephrine for the treatment of anaphylaxis has identified that geriatric patients may be particularly sensitive to the effects of epinephrine. Therefore, epinephrine injection, 0.3 mg should be administered with caution in elderly individuals, who may be at greater risk for developing adverse reactions after epinephrine administration [see Warnings and Precautions (5.5), Overdosage (10)].
- Elderly patients may be at greater risk of developing adverse reactions. (5.5, 8.5)
OVERDOSAGE SECTION
10 OVERDOSAGE
Overdosage of epinephrine may produce extremely elevated arterial pressure, which may result in cerebrovascular hemorrhage, particularly in elderly patients. Overdosage may also result in pulmonary edema because of peripheral vascular constriction together with cardiac stimulation. Treatment consists of rapidly acting vasodilators or alpha-adrenergic blocking drugs and/or respiratory support.
Epinephrine overdosage can also cause transient bradycardia followed by tachycardia, and these may be accompanied by potentially fatal cardiac arrhythmias. Premature ventricular contractions may appear within one minute after injection and may be followed by multifocal ventricular tachycardia (prefibrillation rhythm). Subsidence of the ventricular effects may be followed by atrial tachycardia and occasionally by atrioventricular block. Treatment of arrhythmias consists of administration of a beta-adrenergic blocking drug such as propranolol.
Overdosage sometimes results in extreme pallor and coldness of the skin, metabolic acidosis, and kidney failure. Suitable corrective measures must be taken in such situations.
DESCRIPTION SECTION
11 DESCRIPTION
Epinephrine Injection USP, 0.3 mg and Epinephrine Injection USP, 0.15 mg are single-dose auto-injectors and combination products containing drug and device components.
Each Epinephrine Injection USP, 0.3 mg (Auto-Injector) delivers a single dose of 0.3 mg epinephrine, USP from epinephrine injection USP, 0.3 mg/0.3 mL in a sterile solution.
Each Epinephrine Injection USP, 0.15 mg (Auto-Injector) delivers a single dose of 0.15 mg epinephrine, USP from epinephrine injection USP, 0.15 mg/0.3 mL in a sterile solution.
Each 0.3 mL in the Epinephrine Injection USP, 0.3 mg (Auto-Injector) contains 0.3 mg epinephrine USP, 1.8 mg sodium chloride, 0.4 mg sodium metabisulfite, 0.4 mg sodium tartrate (dihydrate), hydrochloric acid to adjust pH, and water for injection. The pH range is 2.2 to 5.0.
Each 0.3 mL in the Epinephrine Injection USP, 0.15 mg (Auto-Injector) contains 0.15 mg epinephrine USP, 1.8 mg sodium chloride, 0.4 mg sodium metabisulfite, 0.2 mg sodium tartrate (dihydrate), hydrochloric acid to adjust pH, and water for injection. The pH range is 2.2 to 5.0.
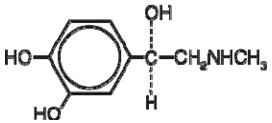
Epinephrine, USP is a sympathomimetic catecholamine. Chemically, epinephrine, USP is (-)-3,4-Dihydroxy-α-[(methylamino)methyl]benzyl alcohol with the following structure:
Epinephrine solution deteriorates rapidly on exposure to air or light, turning pink from oxidation to adrenochrome and brown from the formation of melanin. Replace Epinephrine Injection USP, 0.3 mg and Epinephrine Injection USP, 0.15 mg if the epinephrine solution appears discolored (pinkish or darker than slightly yellow), cloudy, or if it contains a precipitate.
Thoroughly review the patient instructions and operation of Epinephrine Injection USP, 0.3 mg or Epinephrine Injection USP, 0.15 mg with patients and caregivers prior to use [see Patient Counseling Information (17)].
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Epinephrine acts on both alpha- and beta-adrenergic receptors.
12.2 Pharmacodynamics
Through its action on alpha-adrenergic receptors, epinephrine lessens the vasodilation and increased vascular permeability that occurs during anaphylaxis, which can lead to loss of intravascular fluid volume and hypotension.
Through its action on beta-adrenergic receptors, epinephrine causes bronchial smooth muscle relaxation and helps alleviate bronchospasm, wheezing and dyspnea that may occur during anaphylaxis.
Epinephrine also alleviates pruritus, urticaria, and angioedema and may relieve gastrointestinal and genitourinary symptoms associated with anaphylaxis because of its relaxer effects on the smooth muscle of the stomach, intestine, uterus and urinary bladder.
When given subcutaneously or intramuscularly, epinephrine has a rapid onset and short duration of action.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies to evaluate the carcinogenic potential of epinephrine have not been conducted.
Epinephrine and other catecholamines have been shown to have mutagenic potential in vitro. Epinephrine was positive in the Salmonella bacterial reverse mutation assay, positive in the mouse lymphoma assay, and negative in the in vivo micronucleus assay. Epinephrine is an oxidative mutagen based on the E. coli WP2 Mutoxitest bacterial reverse mutation assay. This should not prevent the use of epinephrine where indicated [see Indications and Usage (1)].
The potential for epinephrine to impair reproductive performance has not been evaluated, but epinephrine has been shown to decrease implantation in female rabbits dosed subcutaneously with 1.2 mg/kg/day (40-fold the highest human intramuscular or subcutaneous daily dose) during gestation days 3 to 9.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
Epinephrine Injection USP, 0.3 mg (Auto-Injectors) is supplied with two single-dose pre-filled auto-injectors and one auto-injector trainer device: 0.3 mg/0.3 mL (NDC 0093-5986-27).
Epinephrine Injection USP, 0.15 mg (Auto-Injectors) is supplied with two single-dose pre-filled auto-injectors and one auto-injector trainer device: 0.15 mg/0.3 mL (NDC 0093-5985-27).
Epinephrine Injection USP, 0.3 mg 2-Pack and Epinephrine Injection USP, 0.15 mg 2-Pack also includes a W-clip to clip two auto-injectors together.
Storage and Handling
- Protect from light. Epinephrine, USP is light sensitive and the auto-injector is manufactured from transparent UV stabilized polycarbonate to protect it from light.
- Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature].
- Do not refrigerate.
- Before using, check to make sure the solution in the auto-injector is clear and colorless.
- Replace the auto-injector if the solution is discolored (pinkish or darker than slightly yellow), cloudy, or contains a precipitate.
- Properly dispose all used, unwanted or expired epinephrine injection, 0.3 mg and epinephrine injection, 0.15 mg auto-injectors.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
See FDA-Approved Patient Labeling (Patient Information and Instructions for Use)
A healthcare provider should review the patient instructions and operation of epinephrine injection, 0.3 mg and epinephrine injection, 0.15 mg in detail, with the patient or caregiver.
Epinephrine is essential for the treatment of anaphylaxis. Patients who are at risk of or with a history of severe allergic reactions (anaphylaxis) to insect stings or bites, foods, drugs, and other allergens, as well as idiopathic and exercise-induced anaphylaxis, should be carefully instructed about the circumstances under which epinephrine should be used.
Administration
Instruct patients and/or caregivers in the appropriate use of epinephrine injection, 0.3 mg and epinephrine injection, 0.15 mg should be injected into the middle of the outer thigh (through clothing, if necessary). Each device is a single-use injection. Advise patients to seek immediate medical care in conjunction with administration of epinephrine injection, 0.3 mg and epinephrine injection, 0.15 mg.
Instruct caregivers to hold the leg of young children firmly in place and limit movement prior to and during injection. Lacerations, bent needles, and embedded needles have been reported when epinephrine injection, 0.3 mg and epinephrine injection, 0.15 mg have been injected into the thigh of young children who are uncooperative and kick or move during an injection [see Warnings and Precautions (5.2)].
Instruct patients and/or caregivers to throw away the blue safety release immediately after using epinephrine injection, 0.3 mg and epinephrine injection, 0.15 mg. This small part may pose a choking hazard for children.
Complete patient information, including dosage, directions for proper administration and precautions can be found inside each epinephrine injection, 0.3 mg or epinephrine injection, 0.15 mg carton. A printed label on the surface of epinephrine injection, 0.3 mg and epinephrine injection, 0.15 mg shows instructions for use and a diagram depicting the injection process.
Training
Instruct patients and/or caregivers to use and practice with the Trainer to familiarize themselves with the use of epinephrine injection, 0.3 mg and epinephrine injection, 0.15 mg in an allergic emergency. The Trainer may be used multiple times. A Trainer device is provided in 2-Pack cartons.
Instruct patients and/or caregivers to immediately place the blue safety release back on the Trainer and reset it after practicing. This small part may pose a choking hazard for children.
Adverse Reactions
Epinephrine may produce symptoms and signs that include an increase in heart rate, the sensation of a more forceful heartbeat, palpitations, sweating, nausea and vomiting, difficulty breathing, pallor, dizziness, weakness or shakiness, headache, apprehension, nervousness, or anxiety. These signs and symptoms usually subside rapidly, especially with rest, quiet and recumbency. Patients with hypertension or hyperthyroidism may develop more severe or persistent effects, and patients with coronary artery disease could experience angina. Patients with diabetes may develop increased blood glucose levels following epinephrine administration. Patients with Parkinson’s disease may notice a temporary worsening of symptoms [see Warnings and Precautions (5.5)].
Accidental Injection
Advise patients to seek immediate medical care in the case of accidental injection. Since epinephrine is a strong vasoconstrictor when injected into the digits, hands, or feet, treatment should be directed at vasodilatation if there is such an accidental injection to these areas [see Warnings and Precautions (5.2)].
Serious Infections at the Injection Site
Rare cases of serious skin and soft tissue infections, including necrotizing fasciitis and myonecrosis caused by Clostridia (gas gangrene), have been reported at the injection site following epinephrine injection for anaphylaxis. Advise patients to seek medical care if they develop signs or symptoms of infection, such as persistent redness, warmth, swelling, or tenderness, at the epinephrine injection site [see Warnings and Precautions (5.3)].
Storage and Handling
Instruct patients to inspect the epinephrine solution visually through the clear window of the auto-injector periodically. Replace epinephrine injection, 0.3 mg and epinephrine injection, 0.15 mg if the epinephrine solution appears discolored (pinkish or darker than slightly yellow), cloudy or if it contains a precipitate. Epinephrine is light sensitive and should be protected from light. The auto-injector is not waterproof. Instruct patients that epinephrine injection, 0.3 mg and epinephrine injection, 0.15 mg must be used or properly disposed once the blue safety release is removed or after use [see Storage and Handling (16.2)].
Advise patients and caregivers to give used epinephrine injection, 0.3 mg and epinephrine injection, 0.15 mg auto-injectors to their healthcare provider for inspection and proper disposal.
Advise patients and caregivers to promptly dispose of medicines that are no longer needed. Dispose of expired, unwanted, or unused epinephrine injection, 0.3 mg and epinephrine injection, 0.15 mg auto-injectors in an FDA-cleared sharps container. Instruct patients not to dispose epinephrine injection, 0.3 mg or epinephrine injection, 0.15 mg in their household trash. Instruct patients that if they do not have a FDA-cleared sharps disposal container, they may use a household container that is made of a heavy-duty plastic, can be closed with a tight-fitting and puncture-resistant lid without sharps being able to come out, upright and stable during use, leak-resistant, and properly labeled to warn of hazardous waste inside the container. Inform patients that they can visit the FDA website for additional information on disposal of unused medicines.
Complete patient information, including dosage, directions for proper administration and precautions can be found inside each epinephrine injection, 0.3 mg (Auto-Injector) and epinephrine injection, 0.15 mg (Auto-Injector) carton.
Manufactured For:
Teva Pharmaceuticals USA, Inc.
****North Wales, PA 19454
Rev. D 1/2021
SPL PATIENT PACKAGE INSERT SECTION
PATIENT INFORMATION and INSTRUCTIONS FOR USE
Epinephrine Injection USP, 0.3 mg (Auto-Injector)
Epinephrine Injection USP, 0.3 mg = one dose of 0.3 mg epinephrine USP, 0.3 mg/0.3 mL
Epinephrine Injection USP, 0.15 mg (Auto-Injector)
Epinephrine Injection USP, 0.15 mg = one dose of 0.15 mg epinephrine USP, 0.15 mg/0.3 mL
For allergic emergencies (anaphylaxis)
Patient Information
Read this Patient Information Leaflet carefully before using the epinephrine injection, 0.3 mg (auto-injector) or epinephrine injection, 0.15 mg (auto- injector) and each time you get a refill. There may be new information. You, your parent, caregiver, or others who may be in a position to administer epinephrine injection, 0.3 mg (auto-injector) or epinephrine injection, 0.15 mg (auto-injector), should know how to use it before you have an allergic emergency.
This information does not take the place of talking with your healthcare provider about your medical condition or your treatment.
What is the most important information I should know about epinephrine injection, 0.3 mg and epinephrine injection, 0.15 mg?
1. Epinephrine injection, 0.3 mg and epinephrine injection, 0.15 mg are single-dose automatic injection devices (auto-injectors) that contain epinephrine, a medicine used to treat allergic emergencies (anaphylaxis). Anaphylaxis can be life threatening, can happen within minutes, and can be caused by stinging and biting insects, allergy injections, foods, medicines, exercise, or unknown causes.
Symptoms of anaphylaxis may include:
- trouble breathing
- wheezing
- hoarseness (changes in the way your voice sounds)
- hives (raised reddened rash that may itch)
- severe itching
- swelling of your face, lips, mouth, or tongue
- skin rash, redness, or swelling
- fast heartbeat
- weak pulse
- feeling very anxious
- confusion
- stomach pain
- losing control of urine or bowel movements (incontinence)
- diarrhea or stomach cramps
- dizziness, fainting, or “passing out” (unconsciousness)
2. Always carry your epinephrine injection, 0.3 mg or epinephrine injection,
0.15 mg with you because you may not know when anaphylaxis may happen.
Talk to your healthcare provider if you need additional units to keep at work,
school, or other locations. Tell your family members, caregivers, and others
where you keep your epinephrine injection, 0.3 mg or epinephrine injection,
0.15 mg and how to use it before you need it. You may be unable to speak in an
allergic emergency.
3. When you have an allergic emergency (anaphylaxis)
*Use epinephrine injection, 0.3 mg or epinephrine injection, 0.15 mg right away. ***Get emergency medical help right away.**You may need further medical attention. You may need to use a second epinephrine injection, 0.3 mg or epinephrine injection, 0.15 mg if symptoms continue or recur. Only a healthcare provider should give additional doses of epinephrine if you need more than 2 injections for a single anaphylaxis episode.
What are epinephrine injection, 0.3 mg and epinephrine injection, 0.15 mg?
- Epinephrine injection, 0.3 mg and epinephrine injection, 0.15 mg are disposable, pre-filled auto-injectors used to treat life-threatening, allergic emergencies including anaphylaxis in people who are at risk for or have a history of serious allergic emergencies. Each device contains a single dose of epinephrine.
- Epinephrine injection, 0.3 mg and epinephrine injection, 0.15 mg are for immediate self (or caregiver) administration and do not take the place of emergency medical care. You should get emergency help right away after using epinephrine injection, 0.3 mg and epinephrine injection, 0.15 mg.
- Epinephrine injection, 0.3 mg and epinephrine injection, 0.15 mg are for people who have been prescribed this medicine by their healthcare provider.
- The Epinephrine Injection, 0.3 mg (Auto-Injector) is for patients who weigh 66 pounds or more (30 kilograms or more).
- The Epinephrine Injection, 0.15 mg (Auto-Injector) is for patients who weigh about 33 to 66 pounds (15 to 30 kilograms).
- It is not known if epinephrine injection, 0.3 mg and epinephrine injection, 0.15 mg are safe and effective in children who weigh less than 33 pounds (15 kilograms).
What should I tell my healthcare provider before using the epinephrine injection, 0.3 mg or epinephrine injection, 0.15 mg?
Before you use epinephrine injection, 0.3 mg or epinephrine injection, 0.15 mg, tell your healthcare provider about all your medical conditions, but especially if you:
-
have heart problems or high blood pressure.
-
have diabetes.
-
have thyroid problems.
-
have asthma.
-
have a history of depression.
-
have Parkinson’s disease.
-
are pregnant or plan to become pregnant. It is not known if epinephrine will harm your unborn baby.
-
are breastfeeding or plan to breastfeed. It is not known if epinephrine passes into your breast milk.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Tell your healthcare provider about all of your known allergies.
Especially tell your healthcare provider if you take certain asthma medicines.
Epinephrine injection, 0.3 mg or epinephrine injection, 0.15 mg and other medicines may affect each other, causing side effects. Epinephrine injection, 0.3 mg or epinephrine injection, 0.15 mg may affect the way other medicines work, and other medicines may affect how epinephrine injection, 0.3 mg or epinephrine injection, 0.15 mg work.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
Use your epinephrine injection, 0.3 mg or epinephrine injection, 0.15 mg for treatment of anaphylaxis as prescribed by your healthcare provider, regardless of your medical conditions or the medicines you take.
How should I use epinephrine injection, 0.3 mg and epinephrine injection, 0.15 mg?
- Each epinephrine injection, 0.3 mg (auto-injector) or epinephrine injection, 0.15 mg (auto-injector) contains only 1 dose of medicine (single-dose).
- Epinephrine injection, 0.3 mg and epinephrine injection, 0.15 mg Auto-Injectors deliver a fixed dose of epinephrine. The auto-injectors cannot be reused. Do not try to reuse epinephrine injection, 0.3 mg or epinephrine injection, 0.15 mg after the device has been activated. It is normal for most of the medicine to remain in the auto-injector after the dose has been injected. The dose has been injected if the orange tip is extended and the window is blocked.
- Epinephrine injection, 0.3 mg or epinephrine injection, 0.15 mg should be injected into the middle of your outer thigh (upper leg). It can be injected through your clothing if needed. Do not inject into a vein or into the buttocks, fingers, toes, hands or feet.
- Read the Instructions for Use at the end of this Patient Information Leaflet about the right way to use epinephrine injection, 0.3 mg or epinephrine injection, 0.15 mg.
- Your healthcare provider will show you how to safely use the epinephrine injection, 0.3 mg (auto-injector) or epinephrine injection, 0.15 mg (auto-injector).
- Use your single-dose epinephrine injection, 0.3 mg or epinephrine injection, 0.15 mg exactly as your healthcare provider tells you to use it. You may need to use a second epinephrine injection, 0.3 mg or epinephrine injection, 0.15 mg if symptoms continue or recur. Only a healthcare provider should give additional doses of epinephrine if you need more than 2 injections for a single anaphylaxis episode. *Caution: Never put your thumb, fingers, or hand over the orange tip. Never press or push the orange tip with your thumb, fingers, or hand. The needle comes out of the orange tip. Accidental injection into finger, hands or feet may cause a loss of blood flow to these areas.If an accidental injection happens, go immediately to the nearest emergency room. Tell the healthcare provider where on your body you received the accidental injection.
- Your epinephrine injection, 0.3 mg (auto-injector) and epinephrine injection, 0.15 mg (auto-injector) may come packaged with an Epinephrine Injection Trainer and separate Epinephrine Injection Trainer Instructions for Use.**The Epinephrine Injection Trainer has a grey color.**The grey Epinephrine Injection Trainer contains no medicine and no needle.Keep the Epinephrine Injection Trainer and the real Epinephrine Injection, 0.3 mg and Epinephrine Injection, 0.15 mg Auto-Injectors away from young children. The real Epinephrine Injection, 0.3 mg and Epinephrine Injection, 0.15 mg Auto-Injectors and Epinephrine Injection Trainer are not toys. For young children, use of the Epinephrine Injection Trainer and the real Epinephrine Injection, 0.3 mg and Epinephrine Injection, 0.15 mg Auto-Injectors should be supervised by an adult. Periodically practice with your Epinephrine Injection Trainer before an allergic emergency happens to make sure you are able to safely use the real epinephrine injection, 0.3 mg (auto-injector) and epinephrine injection, 0.15 mg (auto-injector) in an emergency. Always carry your real epinephrine injection, 0.3 mg (auto-injector) or epinephrine injection 0.15 mg (auto-injector) with you in case of an allergic emergency. Additional training resources are available atwww.TevaEpinephrine.com.
- Do not drop the auto-injector. If the auto-injector is dropped, check for damage and leakage. Throw away (dispose of) the auto-injector and replace if damage or leakage is noticed or suspected.
What are the possible side effects of epinephrine injection, 0.3 mg and epinephrine injection, 0.15 mg?
Epinephrine injection, 0.3 mg and epinephrine injection, 0.15 mg may cause serious side effects.
*The epinephrine injection, 0.3 mg or epinephrine injection, 0.15 mg should only be injected into the middle of your outer thigh (upper leg). Do not inject the epinephrine injection, 0.3 mg or epinephrine injection, 0.15 mg into your:
* veins
* buttocks
* fingers, toes, hands, or feet
If you accidentally inject epinephrine injection, 0.3 mg or epinephrine
injection, 0.15 mg into any other part of your body, go to the nearest
emergency room right away. Tell the healthcare provider where on your body you
received the accidental injection.
- Rarely, patients who have used epinephrine injection, 0.3 mg or epinephrine injection, 0.15 mg may develop infections at the injection site within a few days of an injection. Some of these infections can be serious. Call your healthcare provider right away if you have any of the following at an injection site:
- redness that does not go away
- swelling
- tenderness
- the area feels warm to the touch
- Cuts on the skin, bent needles, and needles that remain in the skin after the injection, have happened in young children who do not cooperate and kick or move during an injection. If you inject a young child with epinephrine injection, 0.3 mg or epinephrine injection, 0.15 mg, hold their leg firmly in place before and during the injection to prevent injuries. Ask your healthcare provider to show you how to:
- Hold the young child firmly in place (restrain).
- With 1 hand, quickly twist the yellow cap off the Epinephrine Injection, 0.3 mg auto-injector or the green cap off the Epinephrine Injection, 0.15 mg auto-injector in the direction of the “twist arrow” to remove it.
- Grasp the auto-injector in your fist with the orange tip (needle end) pointing downward.
- With your other hand, pull off the blue safety release.
*If you have certain medical conditions, or take certain medicines, your condition may get worse or you may have longer lasting side effects when you use your epinephrine injection, 0.3 mg or epinephrine injection, 0.15 mg. Talk to your healthcare provider about all your medical conditions.
Common side effects of epinephrine injection, 0.3 mg and epinephrine injection, 0.15 mg include:
- fast, irregular or “pounding” heartbeat
- sweating
- headache
- weakness
- shakiness
- paleness
- feelings of over excitement, nervousness or anxiety
- dizziness
- nausea or vomiting
- breathing problems
These side effects may go away with rest.Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of the epinephrine injection, 0.3 mg or epinephrine injection, 0.15 mg. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store epinephrine injection, 0.3 mg and epinephrine injection, 0.15 mg?
-
Store epinephrine injection, 0.3 mg and epinephrine injection, 0.15 mg at room temperature between 68° to 77°F (20° to 25°C).
-
Protect from light. *Do notexpose to extreme cold or heat. For example,do not store in your vehicle’s glove box anddo not store in the refrigerator or freezer.
-
Examine the contents in the clear window of your auto-injector periodically. The solution should be clear. If the solution is discolored (pinkish or darker than slightly yellow) or contains solid particles, replace the unit.
-
Always protect your epinephrine injection, 0.3 mg (auto-injector) and epinephrine injection, 0.15 mg (auto-injector) from damage and water.
-
The blue safety release helps to prevent accidental injection. Keep the blue safety release on until you need to use epinephrine injection, 0.3 mg or epinephrine injection, 0.15 mg.
-
Your epinephrine injection, 0.3 mg or epinephrine injection, 0.15 mg has an expiration date. Replace it before the expiration date. Throw away (dispose of) expired, unwanted, or unused epinephrine injection, 0.3 mg and epinephrine injection, 0.15 mg in an FDA-cleared sharps disposal container. Do not throw away the epinephrine injection, 0.3 mg or epinephrine injection, 0.15 mg in your household trash. If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- Made of heavy-duty plastic,
- Can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- Upright and stable during use,
- Leak-resistant, and
- Properly labeled to warn of hazardous waste inside the container.
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal
Visit the FDA’s website (https://www.fda.gov/drugs/safe-disposal- medicines/disposal-unused-medicines-what-you-should-know) for more information about how to throw away (dispose of) unused, unwanted or expired medicines.
Keep epinephrine injection, 0.3 mg and epinephrine injection, 0.15 mg and all medicines out of the reach of children.
General information about the safe and effective use of epinephrine injection, 0.3 mg and epinephrine injection, 0.15 mg
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information Leaflet. Do not use the epinephrine injection, 0.3 mg or epinephrine injection, 0.15 mg for a condition for which it was not prescribed. Do not give your epinephrine injection, 0.3 mg or epinephrine injection, 0.15 mg to other people.
This Patient Information Leaflet summarizes the most important information about epinephrine injection, 0.3 mg and epinephrine injection, 0.15 mg. If you would like more information, talk to your healthcare provider. You can ask your pharmacist or healthcare provider for information about epinephrine injection, 0.3 mg and epinephrine injection, 0.15 mg that is written for health professionals.
For more information and video instructions on the use of epinephrine injection, 0.3 mg and epinephrine injection, 0.15 mg go to www.TevaEpinephrine.com or call 1-888-838-2872.
What are the ingredients in epinephrine injection, 0.3 mg and epinephrine injection, 0.15 mg?
Active Ingredients: Epinephrine
Inactive Ingredients: sodium chloride, sodium metabisulfite, sodium tartrate (dihydrate), hydrochloric acid, and water
Important Information
*The Epinephrine Injection, 0.3 mg (Auto-Injector) has a yellow colored label. *The Epinephrine Injection, 0.15 mg (Auto-Injector) has a green colored label. *The Epinephrine Injection Trainer has a grey color and contains no medicine and no needle. *Your auto-injector is designed to work through clothing. *The blue safety release on the Epinephrine Injection, 0.3 mg (Auto-Injector) and Epinephrine Injection, 0.15 mg (Auto-Injector) helps to prevent accidental injection of the device. Do not remove the blue safety release until you are ready to use it. *Choking hazard: The blue safety release is a small part that may become a choking hazard for children. Throw away the blue safety release immediately after using epinephrine injection, 0.3 mg and epinephrine injection, 0.15 mg Auto-Injector. *Only inject into the middle of the outer thigh (upper leg). Never inject into any other part of the body. *Never put your thumb, fingers, or your hand over the orange tip. The needle comes out of the orange tip. *If an accidental injection happens, get emergency medical help right away.
INSTRUCTIONS FOR USE
Epinephrine Injection USP, 0.3 mg (Auto-Injector)
Epinephrine Injection USP, 0.3 mg = one dose of 0.3 mg epinephrine USP, 0.3 mg/0.3 mL
Epinephrine Injection USP, 0.15 mg (Auto-Injector)
**Epinephrine Injection USP, 0.15 mg = one dose of 0.15 mg epinephrine USP, **0.15 mg/0.3 mL
For allergic emergencies (anaphylaxis)
Read this Instructions for Use carefully before you use epinephrine injection, 0.3 mg or epinephrine injection, 0.15 mg. Before you need to use your single- dose epinephrine injection, 0.3 mg or epinephrine injection, 0.15 mg auto- injector, make sure your healthcare provider shows you the right way to use it. Parents, caregivers, and others who may be in a position to administer epinephrine injection, 0.3 mg (auto-injector) or epinephrine injection, 0.15 mg (auto-injector) should also understand how to use it as well. If you have any questions, ask your healthcare provider.
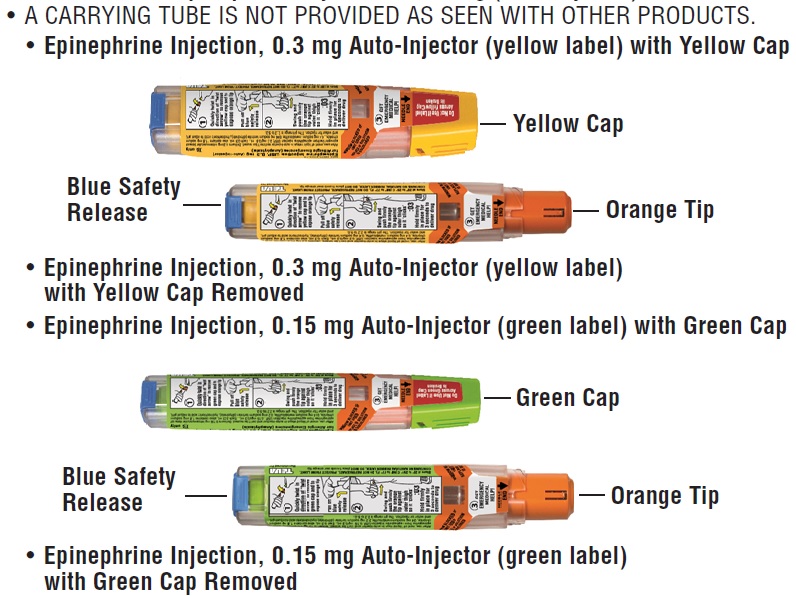
Your Epinephrine Injection, 0.3 mg (Auto-Injector) and Epinephrine Injection, 0.15 mg (Auto-Injector)
A dose of epinephrine injection, 0.3 mg or epinephrine injection, 0.15 mg requires 3 steps: Prepare, Administer and Get emergency medical help
Step 1. Prepare epinephrine injection, 0.3 mg or epinephrine injection, 0.15 mg for injection
|
|
Quickly twist the yellow cap off the epinephrine injection, 0.3 mg auto- injector or the green cap off the epinephrine injection, 0.15 mg auto-injector in the direction of the “twist arrow” to remove it. |
|
|
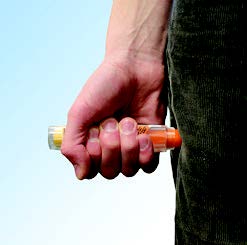
Grasp the auto-injector in your fist with the orange tip (needle end) pointing downward. With your other hand,pull off the blue safety release. |
**Important: The blue safety release is a small part that may become a choking hazard for children. Throw away the blue safety release immediately after using****epinephrine injection,****0.3 mg or epinephrine injection,**0.15 mg.
Note:
- The needle comes out of the orange tip.
- To avoid an accidental injection, never put your thumb, fingers or hand over the orange tip. If an accidental injection happens, get emergency medical help right away.
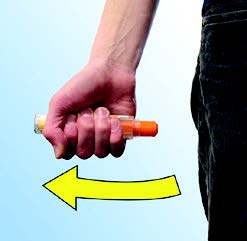
Step 2. Administer epinephrine injection, 0.3 mg or epinephrine injection, 0.15 mg
If you are administering epinephrine injection, 0.3 mg or epinephrine injection, 0.15 mg to a young child, hold the leg firmly in place while administering an injection.
|
|
Place the orange tip against the middle of the outer thigh (upper leg) at a
right angle (perpendicular) to the thigh. The click signals that the injection has started. |
|
|
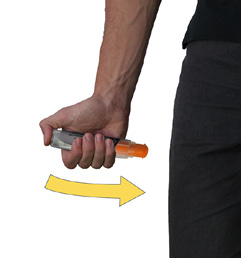
Hold firmly in place for 3 seconds (count slowly 1,2,3). |
|
|
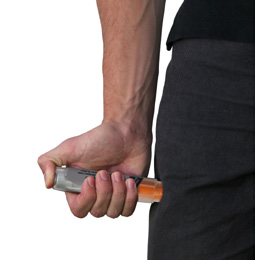
**Remove the auto-injector from the thigh.**The orange tip will extend to cover the needle. If the needle is still visible, do not attempt to reuse it. |
|
|
Massage the injection area for 10 seconds. |
Step 3. Get emergency medical help now. You may need further medical attention. You may need to use a second epinephrine injection, 0.3 mg (auto- injector) or epinephrine injection, 0.15 mg (auto-injector) if symptoms continue or recur.
- Take your used auto-injector with you when you go to see a healthcare provider.
- Tell the healthcare provider that you have received an injection of epinephrine. Show the healthcare provider where you received the injection.
- Give your used epinephrine injection, 0.3 mg (auto-injector) or epinephrine injection, 0.15 mg (auto-injector) to the healthcare provider for inspection and proper disposal.
- Ask for a refill, if needed.
Note:
- Keep the Epinephrine Injection Trainer and the real epinephrine injection, 0.3 mg and epinephrine injection, 0.15 mg Auto-Injectors away from young children. The real epinephrine injection, 0.3 mg and epinephrine injection, 0.15 mg Auto-Injectors and Epinephrine Injection Trainer are not toys. For young children, use of the Epinephrine Injection Trainer and the real epinephrine injection, 0.3 mg and epinephrine injection, 0.15 mg Auto-Injectors should be supervised by an adult.
- A carrying tube is not provided as seen with other products.
- Epinephrine injection, 0.3 mg and epinephrine injection, 0.15 mg are single-dose injectable devices that deliver a fixed dose of epinephrine. The auto-injector cannot be reused. Do not attempt to reuse epinephrine injection after the device has been activated. It is normal for most of the medicine to remain in the auto-injector after the dose is injected. The correct dose has been administered if the orange needle tip is extended and the window is blocked.
- Your epinephrine injection, 0.3 mg (auto-injector) and epinephrine injection, 0.15 mg (auto-injector) may come packaged with an Epinephrine Injection Trainer and separate Epinephrine Injection Trainer Instructions for Use. The Epinephrine Injection Trainer has a grey color. The grey Epinephrine Injection Trainer contains no medicine and no needle. Practice with your Epinephrine Injection Trainer, but always carry your real epinephrine injection, 0.3 mg auto-injector or epinephrine injection, 0.15 mg auto-injector in case of an allergic emergency.
- If you are administering epinephrine injection, 0.3 mg or epinephrine injection, 0.15 mg to a young child, ask your healthcare provider to show you how to (1) hold the young child firmly in place (restrain), (2) with 1 hand, quickly twist the yellow cap off the epinephrine injection, 0.3 mg (auto-injector) or the green cap off the epinephrine injection, 0.15 mg (auto-injector) in the direction of the “twist arrow” to remove it, (3) grasp the auto-injector in your fist with the orange tip (needle end) pointing downward and (4) with your other hand, pull off the blue safety release, and how to properly hold the leg in place while administering a dose.
- Do not try to take the epinephrine injection, 0.3 mg (auto-injector) or epinephrine injection, 0.15 mg (auto-injector) apart.
This Patient Information and Instructions for Use have been approved by the U.S. Food and Drug Administration.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
Manufactured For:
Teva Pharmaceuticals USA, Inc.
****North Wales, PA 19454
Rev. C 1/2021
Epinephrine Injection USP, 0.3 mg (Auto-Injector)
Epinephrine Injection USP, 0.3 mg = one dose of 0.3 mg epinephrine USP, 0.3 mg/0.3 mL
Epinephrine Injection USP, 0.15 mg (Auto-Injector)
Epinephrine Injection USP, 0.15 mg = one dose of 0.15 mg epinephrine USP, 0.15 mg/0.3 mL
TevaEpinephrine.com
Register your Epinephrine Injection 0.3 mg (Auto-Injector) or Epinephrine Injection 0.15 mg (Auto-Injector) atwww.TevaEpinephrine.com and find out more about:
- Free Epinephrine Injection Auto-InjectorRefill Reminder Program. It is important to keep your auto-injector up-to-date.
Register up to 6 Epinephrine Injection 0.3 mg (Auto-Injectors) or Epinephrine Injection 0.15 mg (Auto-Injectors) and receive automaticRefill Reminder Alerts.
- Receive periodic information related to allergies and allergens.
- Instructional Video
For more information about Epinephrine Injection 0.3 mg (Auto-Injector) or Epinephrine Injection 0.15 mg (Auto-Injector) and proper use of the products, call Teva at 1-888-838-2872 or visitwww.TevaEpinephrine.com.
Epinephrine Injection Trainer Instructions for Use
In an emergency: Do not use the grey Trainer. Use your real yellow Epinephrine Injection 0.3 mg Auto-Injector or real green Epinephrine Injection 0.15 mg Auto-Injector.
Important Information
*The Trainer label has a grey color.
*The Trainer contains no medicine and no needle.
Keep the grey Trainer away from young children. The Trainer is not a toy.
Young children should only practice with the Trainer under adult supervision.
- Periodically practice with the grey Trainer before an allergic emergency (anaphylaxis) happens to make sure you are able to safely use the real yellow epinephrine injection 0.3 mg auto-injector or real green epinephrine injection 0.15 mg auto-injector in case of an emergency.
- Always carry your real yellow epinephrine injection 0.3 mg auto-injector or real green epinephrine injection 0.15 mg auto-injector in case of an allergic emergency.
- Small parts like the blue safety release may become a choking hazard for children. Put the blue safety release back on the Trainer and reset it immediately after practicing.
The Epinephrine Injection Trainer
Familiarize yourself with this grey Trainer. Practice until you are comfortable using it.
Your grey Trainer: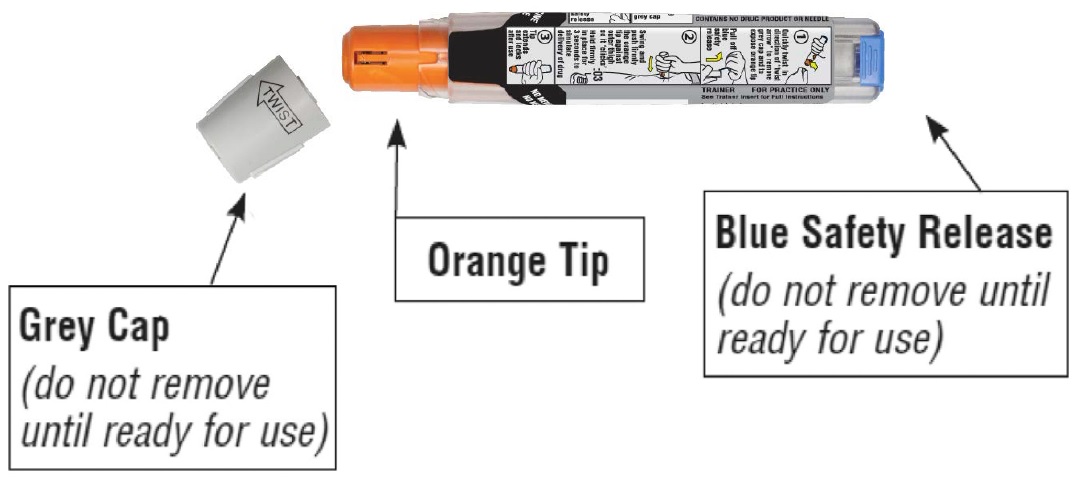
**•**Never put your thumb, other fingers, or hand over the orange tip (below grey safety cap).
• The orange tip is where the needle comes out of your epinephrine injection 0.3 mg auto-injector or epinephrine injection 0.15 mg auto-injector.
|
Practice Instructions |
|
|
1Preparethe Trainer for Simulated Injection *Grasp the grey Trainer in your fist with the orange tip pointing downwardand twist off Grey Cap in the direction of “twist arrow”.
| |
|
2Administerthe Trainer Simulation | ||
|
*If practicing with a young child, hold the leg firmly in place while using the Epinephrine Injection Trainer. 1. Hold the young child firmly in place (restrain). 2. With 1 hand, quickly twist the grey cap off the Epinephrine Injection Trainer in the direction of the “twist arrow” to remove it. 3. Grasp the auto-injector in your fist with the orange tip (needle end) pointing downward. 4. With your other hand, pull off the blue safety release, and how to properly hold the leg to practice so that you will be prepared before an allergic emergency happens. | ||
|
|
Note:
| |
|
|
3To resetthe Trainer
| |
NOTE: With the real yellow Epinephrine Injection 0.3 mg Auto-Injector or real green Epinephrine Injection 0.15 mg Auto-Injector, the orange tip covers the needle after self-injection to help protect you from accidentally sticking yourself or others.
|
Practice Session Information |
In case of an allergic emergency, use the real yellow Epinephrine Injection 0.3 mg Auto-Injector or real green Epinephrine Injection 0.15 mg Auto-Injector and not the grey Trainer.
Follow instructions above. Repeat as often as needed until you are able to self-inject quickly and correctly.
Reread:
- The Trainer Instructions for Use
- The “Patient Information” that comes with your Epinephrine Injection 0.3 mg Auto-Injector or Epinephrine Injection 0.15 mg Auto-Injector
Train others who could help you in an emergency:
• Your parents, caregivers, and others who may be in a position to administer
epinephrine injection 0.3 mg or epinephrine injection 0.15 mg should know how
to help you during an allergic emergency (anaphylaxis). Before an emergency
occurs, have them:
- Practice activating the Trainer
- Read these Trainer Instructions and the “Patient Information”
For more information about the Epinephrine Injection 0.3 mg Auto-Injector and Epinephrine Injection 0.15 mg Auto-Injector and the proper use of the products, go towww.TevaEpinephrine.com.
Caution:
Important differences between the Trainer and your real yellow Epinephrine Injection 0.3 mg (Auto-Injector) or real green Epinephrine Injection 0.15 mg (Auto-Injector)
|
|
|
| |
|
Trainer (Grey) |
Epinephrine Injection 0.3 mg (Yellow) |
Epinephrine Injection 0.15 mg (Green) | |
|
Contains medicine? |
No |
Yes |
Yes |
|
Has needle? |
No |
Yes |
Yes |
|
Comes in Carrier Tube? |
No |
No |
No |
|
Color of label |
Grey |
Yellow |
Green |
|
Has expiration date? |
No |
Yes |
Yes |
|
Can be reused? |
Yes |
No (use only one time) |
No (use only one time) |
|
Okay to remove and replace cap and/or safety release? |
Yes |
No (remove just one time before use) |
No (remove just one time before use) |
|
Pressure needed to hold against thigh? |
Moderate |
Strong |
Strong |
This Trainer Instructions for Use has been approved by the U.S. Food and Drug Administration.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
Manufactured For:
Teva Pharmaceuticals USA, Inc.
****North Wales, PA 19454
Rev. D 1/2021