ELTROMBOPAG
These highlights do not include all the information needed to use ELTROMBOPAG TABLETS safely and effectively. See full prescribing information for ELTROMBOPAG TABLETS. ELTROMBOPAG tablets, for oral use Initial U.S. Approval: 2008
e913cd11-f5fb-4ebe-81c7-0c5626b52cfd
HUMAN PRESCRIPTION DRUG LABEL
Sep 3, 2025
Camber Pharmaceuticals, Inc.
DUNS: 826774775
Products 4
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
ELTROMBOPAG
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (12)
ELTROMBOPAG
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (12)
ELTROMBOPAG
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (12)
ELTROMBOPAG
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Eltrombopag Tablets 12.5 mg Container Label- Annora
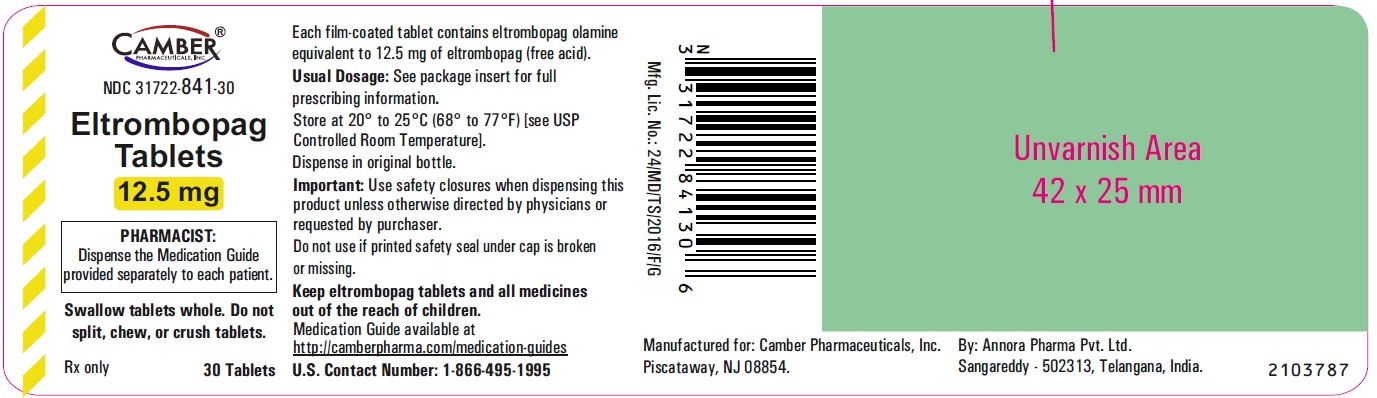
Eltrombopag Tablets 25 mg Container Label- Annora
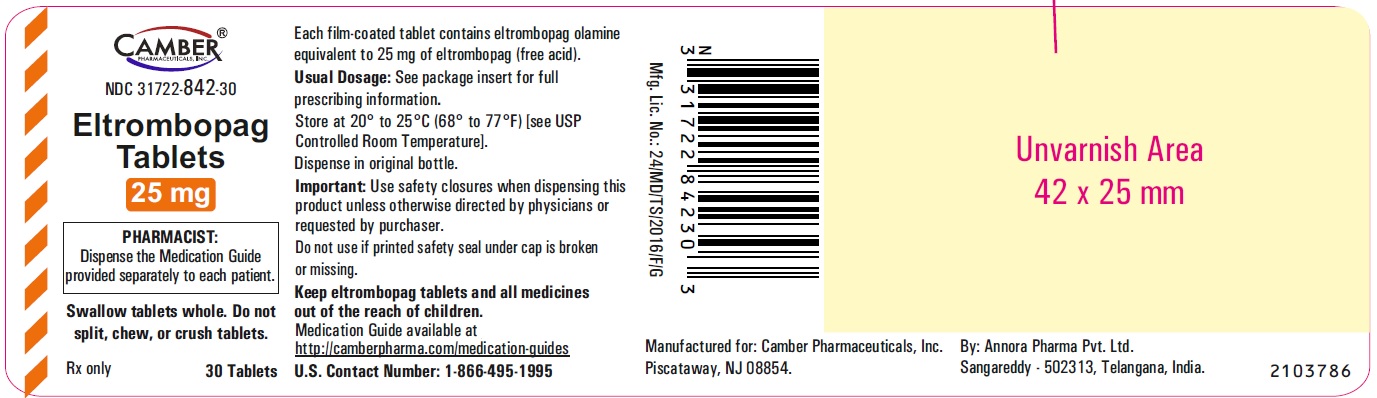
Eltrombopag Tablets 50 mg Container Label- Annora
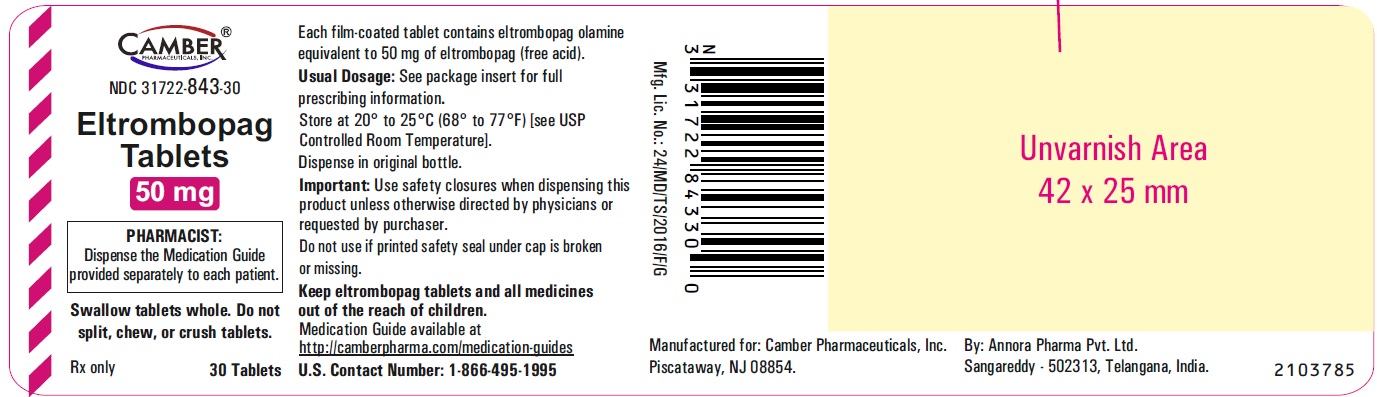
Eltrombopag Tablets 75mg Container Label- Annora
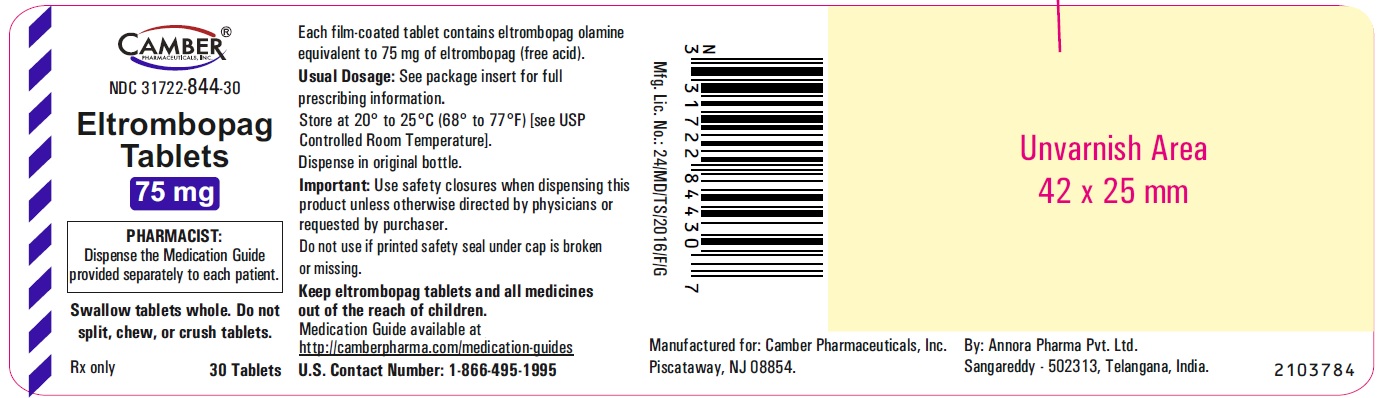
Eltrombopag Tablets 12.5 mg Container Label- Vemagal

Eltrombopag Tablets 25 mg Container Label- Vemagal

Eltrombopag Tablets 50 mg Container Label- Vemagal

Eltrombopag Tablets 75mg Container Label- Vemagal

Eltrombopag Tablets 12.5 mg Container Label- Evaric

Eltrombopag Tablets 25 mg Container Label- Evaric

Eltrombopag Tablets 50 mg Container Label- Evaric

Eltrombopag Tablets 75mg Container Label- Evaric

BOXED WARNING SECTION
**WARNING: RISK FOR HEPATIC DECOMPENSATION IN PATIENTS WITH CHRONIC
HEPATITIS C and RISK OF HEPATOTOXICITY**
See full prescribing information for complete boxed warning.
INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
1.1 Treatment of Thrombocytopenia in Patients With Persistent or Chronic
Immune Thrombocytopenia
Eltrombopag tablets are indicated for the treatment of thrombocytopenia in adult and pediatric patients 1 year and older with persistent or chronic immune thrombocytopenia (ITP) who have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy. Eltrombopag tablets should be used only in patients with ITP whose degree of thrombocytopenia and clinical condition increase the risk for bleeding.
1.2 Treatment of Thrombocytopenia in Patients With Hepatitis C Infection
Eltrombopag tablets are indicated for the treatment of thrombocytopenia in patients with chronic hepatitis C to allow the initiation and maintenance of interferon-based therapy. Eltrombopag tablets should be used only in patients with chronic hepatitis C whose degree of thrombocytopenia prevents the initiation of interferon-based therapy or limits the ability to maintain interferon-based therapy.
1.3 Treatment of Severe Aplastic Anemia
• Eltrombopag tablets are indicated for the treatment of patients with severe aplastic anemia who have had an insufficient response to immunosuppressive therapy.
1.4 Limitations of Use
• Eltrombopag tablets are not indicated for the treatment of patients with
myelodysplastic syndromes (MDS) [see Warnings and Precautions (5.3)].
• Safety and efficacy have not been established in combination with direct-
acting antiviral agents used without interferon for treatment of chronic
hepatitis C infection.
Additional pediatric use information is approved for Novartis Pharmaceuticals Corporation's PROMACTA ®(eltrombopag) tablets. However, due to Novartis Pharmaceuticals Corporation's marketing exclusivity rights, this drug product is not labeled with that information.
Eltrombopag tablet is a thrombopoietin receptor agonist indicated:
• for the treatment of thrombocytopenia in adult and pediatric patients 1 year
and older with persistent or chronic immune thrombocytopenia (ITP) who have
had an insufficient response to corticosteroids, immunoglobulins, or
splenectomy. Eltrombopag tablets should be used only in patients with ITP
whose degree of thrombocytopenia and clinical condition increase the risk for
bleeding. (1.1)
• for the treatment of thrombocytopenia in patients with chronic hepatitis C
to allow the initiation and maintenance of interferon-based therapy.
Eltrombopag tablets should be used only in patients with chronic hepatitis C
whose degree of thrombocytopenia prevents the initiation of interferon-based
therapy or limits the ability to maintain interferon-based therapy. (1.2)
• for the treatment of patients with severe aplastic anemia who have had an
insufficient response to immunosuppressive therapy. (1.3)
Limitations of Use:
• Eltrombopag tablets are not indicated for the treatment of patients with
myelodysplastic syndrome (MDS). (1.4)
• Safety and efficacy have not been established in combination with direct-
acting antiviral agents used without interferon for treatment of chronic
hepatitis C infection. (1.4)
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
None.
None. (4)
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Hepatic Decompensation in Patients With Chronic Hepatitis C
In patients with chronic hepatitis C, eltrombopag in combination with interferon and ribavirin may increase the risk of hepatic decompensation. In two controlled clinical trials in patients with chronic hepatitis C and thrombocytopenia, ascites and encephalopathy occurred more frequently on the arm receiving treatment with eltrombopag plus antivirals (7%) than the placebo plus antivirals arm (4%). Patients with low albumin levels (less than 3.5 g/dL) or Model for End-Stage Liver Disease (MELD) score greater than or equal to 10 at baseline had a greater risk for hepatic decompensation on the arm receiving treatment with eltrombopag plus antivirals. Discontinue eltrombopag if antiviral therapy is discontinued.
5.2 Hepatotoxicity
Eltrombopag may increase the risk of severe and potentially life-threatening
hepatotoxicity [see Adverse Reactions (6.1)]. One patient (< 1%) with ITP
treated with eltrombopag in clinical trials experienced drug-induced liver
injury. Eleven patients (1%) with chronic hepatitis C treated with eltrombopag
in clinical trials experienced drug-induced liver injury.
Treatment of ITP, Chronic Hepatitis C-associated Thrombocytopenia, and
Refractory Severe Aplastic Anemia
Measure serum ALT, AST, and bilirubin prior to initiation of eltrombopag,
every 2 weeks during the dose adjustment phase, and monthly following
establishment of a stable dose [see Drug Interactions (7.5)]. Eltrombopag
inhibits UDP-glucuronosyltransferase (UGT)1A1 and organic anion-transporting
polypeptide (OATP)1B1, which may lead to indirect hyperbilirubinemia. If
bilirubin is elevated, perform fractionation. Evaluate abnormal serum liver
tests with repeat testing within 3 to 5 days. If the abnormalities are
confirmed, monitor serum liver tests weekly until resolved or stabilized.
Discontinue eltrombopag if ALT levels increase to greater than or equal to 3 x
ULN in patients with normal liver function or greater than or equal to 3 x
baseline (or greater than 5 x ULN, whichever is the lower) in patients with
pre-treatment elevations in transaminases and are:
• progressively increasing, or
• persistent for greater than or equal to 4 weeks, or
• accompanied by increased direct bilirubin, or
• accompanied by clinical symptoms of liver injury or evidence for hepatic
decompensation.
If the potential benefit for reinitiating treatment with eltrombopag is considered to outweigh the risk for hepatotoxicity, then consider cautiously reintroducing eltrombopag and measure serum liver tests weekly during the dose adjustment phase. Hepatotoxicity may reoccur if eltrombopag is reinitiated. If liver test abnormalities persist, worsen, or recur, then permanently discontinue eltrombopag.
5.3 Increased Risk of Death and Progression of Myelodysplastic Syndromes to
Acute Myeloid Leukemia
A randomized, double-blind, placebo-controlled, multicenter trial in patients with International Prognostic Scoring System (IPSS) intermediate-1, intermediate-2 or high risk MDS with thrombocytopenia, receiving azacitidine in combination with either eltrombopag (n = 179) or placebo (n = 177) was terminated due to lack of efficacy and safety reasons, including increased progression to acute myeloid leukemia (AML). Patients received eltrombopag or placebo at a starting dose of 200 mg once daily, up to a maximum of 300 mg once daily, in combination with azacitidine for at least six cycles. The incidence of death (overall survival) was 32% (57/179) in the eltrombopag arm versus 29% (51/177) in the placebo arm (HR [95% CI] = 1.42 [0.97, 2.08], showing an increased relative risk of death in this trial by 42% in the eltrombopag arm). The incidence of progression to AML was 12% (21/179) in the eltrombopag arm versus 6% (10/177) in the placebo arm (HR [95% CI] = 2.66 [1.31, 5.41], showing an increased relative risk of progression to AML in this trial by 166% in the eltrombopag arm).
5.4 Thrombotic/Thromboembolic Complications
Thrombotic/thromboembolic complications may result from increases in platelet
counts with eltrombopag. Reported thrombotic/thromboembolic complications
included both venous and arterial events and were observed at low and at
normal platelet counts.
Consider the potential for an increased risk of thromboembolism when
administering eltrombopag to patients with known risk factors for
thromboembolism (e.g., Factor V Leiden, ATIII deficiency, antiphospholipid
syndrome, chronic liver disease). To minimize the risk for
thrombotic/thromboembolic complications, do not use eltrombopag in an attempt
to normalize platelet counts. Follow the dose adjustment guidelines to achieve
and maintain target platelet counts [see Dosage and Administration (2.1, 2.2, 2.3)].
In two controlled clinical trials in patients with chronic hepatitis C and
thrombocytopenia, 3% (31/955) treated with eltrombopag experienced a
thrombotic event compared with 1% (5/484) on placebo. The majority of events
were of the portal venous system (1% in patients treated with eltrombopag
versus less than 1% for placebo).
In a controlled trial in patients with chronic liver disease and
thrombocytopenia not related to ITP undergoing elective invasive procedures (N
= 292), the risk of thrombotic events was increased in patients treated with
75 mg of eltrombopag once daily. Seven thrombotic complications (six patients)
were reported in the group that received eltrombopag and three thrombotic
complications were reported in the placebo group (two patients). All of the
thrombotic complications reported in the group that received eltrombopag were
portal vein thrombosis (PVT). Symptoms of PVT included abdominal pain, nausea,
vomiting, and diarrhea. Five of the six patients in the group that received
eltrombopag experienced a thrombotic complication within 30 days of completing
treatment with eltrombopag and at a platelet count above 200 x 10 9/L. The
risk of portal venous thrombosis was increased in thrombocytopenic patients
with chronic liver disease treated with 75 mg of eltrombopag once daily for 2
weeks in preparation for invasive procedures.
5.5 Cataracts
In the three controlled clinical trials in adults with persistent or chronic
ITP, cataracts developed or worsened in 15 (7%) patients who received 50 mg of
eltrombopag daily and 8 (7%) placebo-group patients. In the extension trial,
cataracts developed or worsened in 11% of patients who underwent ocular
examination prior to therapy with eltrombopag. In the two controlled clinical
trials in patients with chronic hepatitis C and thrombocytopenia, cataracts
developed or worsened in 8% of patients treated with eltrombopag and 5% of
patients treated with placebo.
Cataracts were observed in toxicology studies of eltrombopag in rodents [see Nonclinical Toxicology (13.2)]. Perform a baseline ocular examination prior to
administration of eltrombopag and, during therapy with eltrombopag, regularly
monitor patients for signs and symptoms of cataracts.
5.6 Laboratory Test Interference
Eltrombopagis highly colored and can cause patient sample discoloration, which can interfere with some clinical laboratory tests. Inaccurate test results that are inconsistent with clinical observations may occur for multiple clinical chemistry tests including bilirubin and creatinine. In addition, other lab tests may be impacted, including but not limited to total protein and albumin, and incorrect test results may be generated if there is eltrombopag in the patient’s specimen. Communicate to the lab conducting the testing if your patient is taking eltrombopag. Re-testing using other methods may also help in determining the validity of the test results [see Drug Interactions (7.5)].
Additional pediatric use information is approved for Novartis Pharmaceuticals Corporation's PROMACTA ®(eltrombopag) tablets. However, due to Novartis Pharmaceuticals Corporation's marketing exclusivity rights, this drug product is not labeled with that information.
• Hepatotoxicity:Monitor liver function before and during therapy. (5.2)
• Increased Risk of Death and Progression of Myelodysplastic Syndromes to
Acute Myeloid Leukemia.(5.3)
• Thrombotic/Thromboembolic Complications:Portal vein thrombosis has been
reported in patients with chronic liver disease receiving eltrombopag. Monitor
platelet counts regularly. (5.4)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following clinically significant adverse reactions associated with
eltrombopag are described in other sections.
• Hepatic Decompensation in Patients with Chronic Hepatitis C [see Warnings and Precautions (5.1)]
• Hepatotoxicity [see Warnings and Precautions (5.2)]
• Increased Risk of Death and Progression of Myelodysplastic Syndromes to
Acute Myeloid Leukemia [see Warnings and Precautions (5.3)]
• Thrombotic/Thromboembolic Complications [see Warnings and Precautions (5.4)]
• Cataracts [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse
reaction rates observed in the clinical trials of a drug cannot be directly
compared with rates in the clinical trials of another drug and may not reflect
the rates observed in practice.
Persistent or Chronic Immune Thrombocytopenia
Adults:In clinical trials, hemorrhage was the most common serious adverse
reaction and most hemorrhagic reactions followed discontinuation of
eltrombopag. Other serious adverse reactions included
thrombotic/thromboembolic complications [see Warnings and Precautions (5.4)].
The data described below reflect exposure of eltrombopag to patients with
persistent or chronic ITP aged 18 to 85 years, of whom 66% were female, in
three placebo-controlled trials and one open-label extension trial [see Clinical Studies (14.1)]. Eltrombopag was administered to 330 patients for at
least 6 months and 218 patients for at least 1 year.
Table 8 presents the most common adverse drug reactions (experienced by
greater than or equal to 3% of patients receiving eltrombopag) from the three
placebo-controlled trials, with a higher incidence in eltrombopag versus
placebo.
Table 8. Adverse Reactions (≥ 3%) From Three Placebo-controlled Trials in Adults With Persistent or Chronic Immune Thrombocytopenia
|
Adverse reaction |
Eltrombopag 50 mg |
Placebo |
|
Nausea |
9 |
3 |
|
Diarrhea |
9 |
7 |
|
Upper respiratory tract infection |
7 |
6 |
|
Vomiting |
6 |
< 1 |
|
Urinary tract infection a |
5 |
4 |
|
Increased ALT |
5 |
3 |
|
Myalgia |
5 |
2 |
|
Oropharyngeal pain |
4 |
3 |
|
Increased AST |
4 |
2 |
|
Pharyngitis |
4 |
2 |
|
Back pain |
3 |
2 |
|
Influenza |
3 |
2 |
|
Paresthesia |
3 |
2 |
|
Rash |
3 |
2 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase.
aIncludes PTs of urinary tract infection, cystitis, urinary tract infection
bacterial, and bacteriuria.
In the three controlled clinical persistent or chronic ITP trials, alopecia,
musculoskeletal pain, blood alkaline phosphatase increased, and dry mouth were
the adverse reactions reported in 2% of patients treated with eltrombopag and
in no patients who received placebo.
Among 302 patients with persistent or chronic ITP who received eltrombopag in
the single-arm extension trial, the adverse reactions occurred in a pattern
similar to that seen in the placebo-controlled trials. Table 9 presents the
most common treatment-related adverse reactions (experienced by greater than
or equal to 3% of patients receiving eltrombopag) from the extension trial.
Table 9. Treatment-related Adverse Reactions (≥3%) From Extension Trial in Adults With Persistent or Chronic Immune Thrombocytopenia
|
Adverse reaction |
Eltrombopag 50 mg |
|
Headache |
10 |
|
ALT increased |
5 |
|
AST increased |
5 |
|
Cataract |
5 |
|
Fatigue |
5 |
|
Blood bilirubin increased |
4 |
|
Nausea |
4 |
|
Hyperbilirubinemia |
3 |
|
Diarrhea |
3 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase.
In the three controlled persistent or chronic ITP trials, serum liver test
abnormalities (predominantly Grade 2 or less in severity) were reported in 11%
and 7% of patients for eltrombopag and placebo, respectively. Four patients
(1%) treated with eltrombopag and three patients in the placebo group (2%)
discontinued treatment due to hepatobiliary laboratory abnormalities.
Seventeen of the patients treated with eltrombopag in the controlled trials
with hepatobiliary laboratory abnormalities were re-exposed to eltrombopag in
the extension trial. Eight of these patients again experienced liver test
abnormalities (less than or equal to Grade 3) resulting in discontinuation of
eltrombopag in one patient. In the extension persistent or chronic ITP trial,
six additional patients had eltrombopag discontinued due to liver test
abnormalities (less than or equal to Grade 3).
In the three controlled persistent or chronic ITP trials, cataracts developed
or worsened in 7% of patients treated with eltrombopag and 7% of patients in
the placebo group. All patients had documented, preexisting risk factors for
cataractogenesis, including corticosteroid use. In the extension trial,
cataracts developed or worsened in 11% of patients who underwent ocular
examination prior to therapy with eltrombopag. Seventy-two percent of patients
had preexisting risk factors, including corticosteroid use.
The safety of eltrombopag was also assessed in all patients treated in 7 adult
persistent or chronic ITP clinical trials (N = 763 eltrombopag -treated
patients and 179 placebo-treated patients). Thromboembolic events were
reported in 6% of eltrombopag-treated patients versus 0% of placebo-treated
patients and thrombotic microangiopathy with acute renal failure was reported
in < 1% of eltrombopag-treated patients versus 0% of placebo-treated patients.
In a placebo-controlled trial of eltrombopag in patients with chronic liver
disease and thrombocytopenia not related to ITP, six patients treated with
eltrombopag and one patient in the placebo group developed portal vein
thromboses [see Warnings and Precautions (5.4)].
Pediatric Patients:The data described below reflect median exposure to
eltrombopag of 91 days for 107 pediatric patients (aged 1 to 17 years) with
persistent or chronic ITP, of whom 53% were female, across the randomized
phase of two placebo-controlled trials.
Table 10 presents the most common adverse drug reactions (experienced by
greater than or equal to 3% of pediatric patients 1 year and older receiving
eltrombopag) across the two placebo-controlled trials, with a higher incidence
for eltrombopag versus placebo.
Table 10. Adverse Reactions (≥ 3%) With a Higher Incidence for Eltrombopag Versus Placebo From Two Placebo-controlled Trials in Pediatric Patients 1 Year and Older With Persistent or Chronic Immune Thrombocytopenia
|
Adverse reaction |
Eltrombopag |
Placebo |
|
Upper respiratory tract infection |
17 |
6 |
|
Nasopharyngitis |
12 |
4 |
|
Cough |
9 |
0 |
|
Diarrhea |
9 |
2 |
|
Pyrexia |
9 |
8 |
|
Abdominal pain |
8 |
4 |
|
Oropharyngeal pain |
8 |
2 |
|
Toothache |
6 |
0 |
|
ALT increased a |
6 |
0 |
|
Rash |
5 |
2 |
|
AST increased |
4 |
0 |
|
Rhinorrhea |
4 |
0 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase.
aIncludes adverse reactions or laboratory abnormalities > 3 x ULN.
In the two controlled clinical persistent or chronic ITP trials, cataracts
developed or worsened in 2 (1%) patients treated with eltrombopag. Both
patients had received chronic oral corticosteroids, a risk factor for
cataractogenesis.
Chronic Hepatitis C-associated Thrombocytopenia:In the two placebo-controlled
trials, 955 patients with chronic hepatitis C-associated thrombocytopenia
received eltrombopag. Table 11 presents the most common adverse drug reactions
(experienced by greater than or equal to 10% of patients receiving eltrombopag
compared with placebo).
Table 11. Adverse Reactions (≥ 10% and Greater Than Placebo) From Two
Placebo-controlled Trials in Adults With Chronic Hepatitis C
|
Adverse reaction |
Eltrombopag + Peginterferon/Ribavirin |
Placebo + Peginterferon/Ribavirin |
|
Anemia |
40 |
35 |
|
Pyrexia |
30 |
24 |
|
Fatigue |
28 |
23 |
|
Headache |
21 |
20 |
|
Nausea |
19 |
14 |
|
Diarrhea |
19 |
11 |
|
Decreased appetite |
18 |
14 |
|
Influenza-like illness |
18 |
16 |
|
Insomnia a |
16 |
15 |
|
Asthenia |
16 |
13 |
|
Cough |
15 |
12 |
|
Pruritus |
15 |
13 |
|
Chills |
14 |
9 |
|
Myalgia |
12 |
10 |
|
Alopecia |
10 |
6 |
|
Peripheral edema |
10 |
5 |
aIncludes PTs of insomnia, initial insomnia, and poor quality sleep.
Rash was reported in 9% and 7% of patients receiving eltrombopag and placebo,
respectively.
In the two controlled clinical trials in patients with chronic hepatitis C,
hyperbilirubinemia was reported in 8% of patients receiving eltrombopag
compared with 3% for placebo. Total bilirubin greater than or equal to 1.5 x
ULN was reported in 76% and 50% of patients receiving eltrombopag and placebo,
respectively. ALT or AST greater than or equal to 3 x ULN was reported in 34%
and 38% of patients for eltrombopag and placebo, respectively.
In the two controlled clinical trials in patients with chronic hepatitis C,
cataracts developed or worsened in 8% of patients treated with eltrombopag and
5% of patients treated with placebo.
The safety of eltrombopag was also assessed in all patients treated with
eltrombopag in the two controlled trials, including patients who initially
received eltrombopag in the pre-antiviral treatment phase of the trial and
were later randomized to the placebo arm (N = 1520 eltrombopag-treated
patients). Hepatic failure was reported in 0.8% of eltrombopag-treated
patients and 0.4% of placebo-treated patients.
Severe Aplastic Anemia:
Refractory Severe Aplastic Anemia
In the single-arm, open-label trial, 43 patients with refractory severe
aplastic anemia received eltrombopag. Eleven patients (26%) were treated for
greater than 6 months and 7 patients (16%) were treated for greater than 1
year. The most common adverse reactions (greater than or equal to 20%) were
nausea, fatigue, cough, diarrhea, and headache.
Table 13. Adverse Reactions (≥ 10%) From One Open-label Trial in Adults With
Refractory Severe Aplastic Anemia
|
Adverse reaction |
Eltrombopag |
|
Nausea |
33 |
|
Fatigue |
28 |
|
Cough |
23 |
|
Diarrhea |
21 |
|
Headache |
21 |
|
Pain in extremity |
19 |
|
Pyrexia |
14 |
|
Dizziness |
14 |
|
Oropharyngeal pain |
14 |
|
Abdominal pain |
12 |
|
Muscle spasms |
12 |
|
Transaminases increased |
12 |
|
Arthralgia |
12 |
|
Rhinorrhea |
12 |
Rash and hyperbilirubinemia were reported in 7% of patients; cataract was
reported in 2% of patients.
In this trial, concurrent ALT or AST greater than 3 x ULN with total bilirubin
greater than 1.5 x ULN were reported in 5% of patients. Total bilirubin
greater than 1.5 x ULN occurred in 14% of patients.
In this trial, patients had bone marrow aspirates evaluated for cytogenetic
abnormalities. Eight patients had a new cytogenetic abnormality reported on
therapy, including 5 patients who had complex changes in chromosome 7.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use
of eltrombopag. Because these reactions are reported voluntarily from a
population of uncertain size, it is not always possible to reliably estimate
the frequency or establish a causal relationship to drug exposure.
Skin and Subcutaneous Tissue Disorders:Skin discoloration, including
hyperpigmentation and skin yellowing.
Additional pediatric use information is approved for Novartis Pharmaceuticals
Corporation's PROMACTA ®(eltrombopag) tablets. However, due to Novartis
Pharmaceuticals Corporation's marketing exclusivity rights, this drug product
is not labeled with that information.
Across all indications, the most common adverse reactions (≥ 20% in any
indication) were: anemia, nausea, pyrexia, alanine aminotransferase increased,
cough, fatigue, headache, and diarrhea. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Annora Pharma Private Limited
at 1-866-495-1995 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Polyvalent Cations (Chelation)
Eltrombopag chelates polyvalent cations (such as iron, calcium, aluminum,
magnesium, selenium, and zinc) in foods, mineral supplements, and antacids.
Take eltrombopag at least 2 hours before or 4 hours after any medications or
products containing polyvalent cations, such as antacids, dairy products, and
mineral supplements to avoid significant reduction in absorption of
eltrombopag due to chelation [see Dosage and Administration (2.4), Clinical Pharmacology (12.3)].
7.2 Transporters
Use caution when concomitantly administering eltrombopag and drugs that are substrates of OATP1B1 (e.g., atorvastatin, bosentan, ezetimibe, fluvastatin, glyburide, olmesartan, pitavastatin, pravastatin, rosuvastatin, repaglinide, rifampin, simvastatin acid, SN-38 [active metabolite of irinotecan], valsartan) or breast cancer resistance protein (BCRP) (e.g., imatinib, irinotecan, lapatinib, methotrexate, mitoxantrone, rosuvastatin, sulfasalazine, topotecan). Monitor patients closely for signs and symptoms of excessive exposure to the drugs that are substrates of OATP1B1 or BCRP and consider reduction of the dose of these drugs, if appropriate. In clinical trials with eltrombopag, a dose reduction of rosuvastatin by 50% was recommended.
7.3 Protease Inhibitors
HIV Protease Inhibitors:No dose adjustment is recommended when eltrombopag is
coadministered with lopinavir/ritonavir (LPV/RTV). Drug interactions with
other HIV protease inhibitors have not been evaluated.
Hepatitis C Virus Protease Inhibitors:No dose adjustments are recommended when
eltrombopag is coadministered with boceprevir or telaprevir. Drug interactions
with other hepatitis C virus (HCV) protease inhibitors have not been
evaluated.
7.4 Peginterferon Alfa-2a/b Therapy
No dose adjustments are recommended when eltrombopag is coadministered with peginterferon alfa-2a (PEGASYS ®) or -2b (PEGINTRON ®).
7.5 Interference with Clinical Laboratory Tests
Eltrombopag is highly colored and can cause patient sample discoloration,
which is reported to interfere with some clinical laboratory tests, including,
but not limited to bilirubin and creatinine.
Bilirubin Testing:Eltrombopag can cause both positive and negative
interference with bilirubin assays. If the laboratory results for bilirubin
are inconsistent with clinical observations, further evaluation of liver
function should be performed to clarify the clinical status of the patient.
Evaluating contemporaneous aminotransferase values (AST, ALT) may help
determine the validity of normal total bilirubin levels in the presence of
clinical jaundice.
Creatinine Testing:Eltrombopag can cause positive interference with creatinine
measurements, leading to falsely elevated creatinine levels. In the event of
an unexpected serum creatinine test result, further evaluation of renal
function should be performed. Blood urea should be evaluated if serum
creatinine is unexpectedly high.
Communicate to the lab conducting testing if the patient is taking
eltrombopag. Re-testing using other methods may also help in determining the
validity of the test results.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Eltrombopag is a TPO-receptor agonist that interacts with the transmembrane domain of the human TPO-receptor (also known as cMpl) and initiates signaling cascades that induce proliferation and differentiation of megakaryocytes leading to increased platelet production.
12.2 Pharmacodynamics
In clinical trials, treatment with eltrombopag resulted in dose-dependent
increases in platelet counts following repeated (daily) dosing. The increase
in platelet counts reached a maximum approximately two weeks after the
initiation of dosing, and returned to baseline within approximately two weeks
after the last dose of eltrombopag.
Cardiac Electrophysiology
At doses up to 150 mg (the maximum recommended dose) daily for 5 days,
eltrombopag did not prolong the QT/QTc interval to any relevant extent.
12.3 Pharmacokinetics
Eltrombopag demonstrated a dose-proportional increase in exposure between
doses of 50 to 150 mg/day in healthy adult subjects. Eltrombopag AUC was
approximately 1.7-fold higher in patients with persistent or chronic ITP and
approximately 2.8-fold higher in patients with HCV compared to healthy
subjects. Steady-state was achieved after approximately 1 week of once daily
treatment, with geometric mean accumulation ratio of 1.56 (90% confidence
interval 1.20, 1.63) at 75 mg/day. Eltrombopag for oral suspension delivered
22% higher plasma AUC 0-INFthan the tablet formulation.
Absorption
Eltrombopag is absorbed with a peak concentration occurring 2 to 6 hours after
oral administration. Oral absorption of drug-related material following
administration of a single 75 mg solution dose was estimated to be at least
52%.
Effect of Food
A standard high-fat breakfast (876 calories, 52 g fat, 71 g carbohydrate, 34 g
protein, and 427 mg calcium) significantly decreased plasma eltrombopag AUC
0-INFby approximately 59% and C maxby 65% and delayed T maxby 1 hour. The
decrease in exposure is primarily due to the high calcium content.
A meal low in calcium (≤ 50 mg calcium) did not significantly impact plasma
eltrombopag exposure, regardless of calorie and fat content.
Distribution
The concentration of eltrombopag in blood cells is approximately 50% to 79% of
plasma concentrations based on a radiolabel study. In vitrostudies suggest
that eltrombopag is highly bound to human plasma proteins (greater than 99%).
Eltrombopag is a substrate of BCRP, but is not a substrate for P-glycoprotein
(P-gp) or OATP1B1.
Elimination
The plasma elimination half-life of eltrombopag is approximately 21 to 32
hours in healthy subjects and 26 to 35 hours in patients with ITP.
Metabolism:Absorbed eltrombopag is extensively metabolized, predominantly
through pathways, including cleavage, oxidation, and conjugation with
glucuronic acid, glutathione, or cysteine. In vitrostudies suggest that CYP1A2
and CYP2C8 are responsible for the oxidative metabolism of eltrombopag. UGT1A1
and UGT1A3 are responsible for the glucuronidation of eltrombopag.
Excretion:The predominant route of eltrombopag excretion is via feces (59%),
and 31% of the dose is found in the urine. Unchanged eltrombopag in feces
accounts for approximately 20% of the dose; unchanged eltrombopag is not
detectable in urine.
Specific Populations
Ethnicity
Eltrombopag concentrations in East-/Southeast-Asian ancestry patients with ITP
or chronic hepatitis C were 50% to 55% higher compared with non-Asian subjects
[see Dosage and Administration (2.1, 2.3)].
Eltrombopag exposure in healthy African-American subjects was approximately
40% higher than that observed in Caucasian subjects in one clinical
pharmacology trial and similar in three other clinical pharmacology trials.
The effect of African-American ethnicity on exposure and related safety and
efficacy of eltrombopag has not been established.
Hepatic Impairment
Following a single dose of eltrombopag (50 mg), plasma eltrombopag AUC
0-INFwas 41% higher in patients with mild hepatic impairment (Child-Pugh class
A) compared with subjects with normal hepatic function. Plasma eltrombopag AUC
0-INFwas approximately 2-fold higher in patients with moderate (Child-Pugh
class B) and severe hepatic impairment (Child-Pugh class C) compared with
subjects with normal hepatic function. The half-life of eltrombopag was
prolonged 2-fold in these patients. This clinical trial did not evaluate
protein-binding effects.
Chronic Liver Disease
Following repeat doses of eltrombopag in patients with thrombocytopenia and
with chronic liver disease, mild hepatic impairment resulted in an 87% to 110%
higher plasma eltrombopag AUC (0-τ)and moderate hepatic impairment resulted in
approximately 141% to 240% higher plasma eltrombopag AUC (0-τ)values compared
with patients with normal hepatic function. The half-life of eltrombopag was
prolonged 3-fold in patients with mild hepatic impairment and 4-fold in
patients with moderate hepatic impairment. This clinical trial did not
evaluate protein-binding effects.
Chronic Hepatitis C
Patients with chronic hepatitis C treated with eltrombopag had higher plasma
AUC (0-τ)values as compared with healthy subjects, and AUC (0-τ)increased with
increasing Child-Pugh score. Patients with chronic hepatitis C and mild
hepatic impairment had approximately 100% to 144% higher plasma AUC
(0-τ)compared with healthy subjects. This clinical trial did not evaluate
protein-binding effects.
Renal Impairment
Following a single dose of eltrombopag (50 mg), the average total plasma
eltrombopag AUC 0-INFwas 32% to 36% lower in subjects with mild (estimated
creatinine clearance (CLCr) by Cockcroft-Gault equation: 50 to 80 mL/min), to
moderate (CLCr of 30 to 49 mL/min) renal impairment and 60% lower in subjects
with severe (CLCr less than 30 mL/min) renal impairment compared with healthy
subjects. The effect of renal impairment on unbound (active) eltrombopag
exposure has not been assessed.
Pediatric Patients
The pharmacokinetics of eltrombopag have been evaluated in 168 pediatric
patients 1 year and older with ITP dosed once daily in two trials. Plasma
eltrombopag apparent clearance following oral administration (CL/F) increased
with increasing body weight. East-/Southeast-Asian pediatric patients with ITP
had approximately 43% higher plasma eltrombopag AUC (0-τ)values as compared
with non-Asian patients.
Plasma eltrombopag AUC (0-τ)and C maxin pediatric patients aged 12 to 17 years was similar to that observed in adults. The pharmacokinetic parameters of eltrombopag in pediatric patients with ITP are shown in Table 15.
Table 15. Geometric Mean (95% CI) Steady-state Plasma Eltrombopag Pharmacokinetic Parametersain Patients With ITP (Normalized to a Once-daily 50 mg Dose)
|
Age |
C****maxb |
AUC**(0-**τ)b |
|
Adults (n = 108) |
7.03 (6.44, 7.68) |
101 (91.4, 113) |
|
12 to 17 years (n = 62) |
6.80 (6.17, 7.50) |
103 (91.1, 116) |
|
6 to 11 years (n = 68) |
10.3 (9.42, 11.2) |
153 (137, 170) |
|
1 to 5 years (n = 38) |
11.6 (10.4, 12.9) |
162 (139, 187) |
aPK parameters presented as geometric mean (95% CI).
bBased on population PK post-hoc estimates.
Drug Interaction Studies
Clinical Studies
Effect of Drugs on Eltrombopag
Effect of Polyvalent Cation-containing Antacids on Eltrombopag:
The coadministration of a single dose of eltrombopag (75 mg) with a polyvalent
cation-containing antacid (1,524 mg aluminum hydroxide, 1,425 mg magnesium
carbonate, and sodium alginate) decreased plasma eltrombopag AUC 0-INFand C
maxby approximately 70%. The contribution of sodium alginate to this
interaction is not known.
Effect of HIV Protease Inhibitors on Eltrombopag:
The coadministration of repeat-dose lopinavir 400 mg/ritonavir 100 mg (twice
daily) with a single dose of eltrombopag (100 mg) decreased plasma eltrombopag
AUC 0-INFby 17%.
Effect of HCV Protease Inhibitors on Eltrombopag:
The coadministration of repeat-dose telaprevir (750 mg every 8 hours) or
boceprevir (800 mg every 8 hours) with a single dose of eltrombopag (200 mg)
to healthy adult subjects in a clinical trial did not alter plasma eltrombopag
AUC 0-INFor C maxto a significant extent.
Effect of Cyclosporine on Eltrombopag:
The coadministration of a single dose of eltrombopag (50 mg) with a single
dose of an OATP and BCRP inhibitor cyclosporine (200 mg or 600 mg) decreased
plasma eltrombopag AUC 0-INFby 18% to 24% and C maxby 25% to 39%.
Effect of Pegylated Interferon alfa-2a + Ribavirin and Pegylated Interferon
alfa-2b + Ribavirin on Eltrombopag:
The presence of pegylated interferon alfa + ribavirin therapy did not
significantly affect the clearance of eltrombopag.
Effect of Eltrombopag on Other Drugs
Effect of Eltrombopag on Cytochrome P450 Enzymes Substrates:
The coadministration of multiple doses of eltrombopag (75 mg once daily for 7
days) did not result in the inhibition or induction of the metabolism of a
combination of probe substrates for CYP1A2 (caffeine), CYP2C19 (omeprazole),
CYP2C9 (flurbiprofen), or CYP3A4 (midazolam) in humans.
Effect of Eltrombopag on Rosuvastatin:
The coadministration of multiple doses of eltrombopag (75 mg once daily for 5
days) with a single dose of rosuvastatin (OATP1B1 and BCRP substrate; 10 mg)
increased plasma rosuvastatin AUC 0-INFby 55% and C maxby 103%.
Effect of Eltrombopag on HCV Protease Inhibitors:
The coadministration of repeat-dose telaprevir (750 mg every 8 hours) or
boceprevir (800 mg every 8 hours) with a single dose of eltrombopag (200 mg)
to healthy adult subjects in a clinical trial did not alter plasma telaprevir
or boceprevir AUC 0-INFor C maxto a significant extent.
In vitroStudies
Eltrombopag Effect on Metabolic Enzymes
Eltrombopag has demonstrated the potential to inhibit CYP2C8, CYP2C9, UGT1A1,
UGT1A3, UGT1A4, UGT1A6, UGT1A9, UGT2B7, and UGT2B15.
Eltrombopag Effect on Transporters
Eltrombopag has demonstrated the potential to inhibit OATP1B1 and BCRP.
Additional pediatric use information is approved for Novartis Pharmaceuticals
Corporation's PROMACTA ®(eltrombopag) tablets. However, due to Novartis
Pharmaceuticals Corporation's marketing exclusivity rights, this drug product
is not labeled with that information.
SPL UNCLASSIFIED SECTION
MEDICATION GUIDE
|
Eltrombopag |
|
What is the most important information I should know about eltrombopag
tablets? See “What are the possible side effects of eltrombopag tablets?” for other side effects of eltrombopag tablets. |
|
What are eltrombopag tablets? |
|
Before you take eltrombopag tablets, tell your healthcare provider about all
of your medical conditions, including if you: • are breastfeeding or plan to breastfeed. You should not breastfeed during
your treatment with eltrombopag tablets. Talk to your healthcare provider
about the best way to feed your baby during this time. Especially tell your healthcare provider if you take: |
|
How should I take eltrombopag tablets? |
|
What should I avoid while taking eltrombopag tablets? |
|
What are the possible side effects of eltrombopag tablets? •**High platelet counts and higher risk for blood clots.**Your risk of
getting a blood clot is increased if your platelet count is too high during
treatment with eltrombopag tablets. Your risk of getting a blood clot may also
be increased during treatment with eltrombopag tablets if you have normal or
low platelet counts. You may have severe problems or die from some forms of
blood clots, such as clots that travel to the lungs or that cause heart
attacks or strokes. Your healthcare provider will check your blood platelet
counts, and change your dose or stop eltrombopag tablets if your platelet
counts get too high. Tell your healthcare provider right away if you have
signs and symptoms of a blood clot in the leg, such as swelling, pain, or
tenderness in your leg. The most common side effects of eltrombopag tablets in adults and children
include: |
|
How should I store eltrombopag tablets? |
|
General information about the safe and effective use of eltrombopag
tablets |
|
What are the ingredients in eltrombopag tablets? Medication Guide available at http://camberpharma.com/medication-guides Manufactured for: By: Annora Pharma Pvt. Ltd. By:HETERO****TM (or) By: Evaric Pharmaceuticals Inc. |
This Medication Guide has been approved by the U.S. Food and Drug
Administration
Revised: 08/2025
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Persistent or Chronic ITP
Adults:The efficacy and safety of eltrombopag in adult patients with
persistent or chronic ITP were evaluated in three randomized, double-blind,
placebo-controlled trials and in an open-label extension trial.
In Study TRA100773B and Study TRA100773A (referred to as Study 773B and Study
773A, respectively [NCT00102739]), patients who had completed at least one
prior ITP therapy and who had a platelet count less than 30 x 10 9/L were
randomized to receive either eltrombopag or placebo daily for up to 6 weeks,
followed by 6 weeks off therapy. During the trials, eltrombopag or placebo was
discontinued if the platelet count exceeded 200 x 10 9/L.
The median age of the patients was 50 years and 60% were female. Approximately
70% of the patients had received at least 2 prior ITP therapies (predominantly
corticosteroids, immunoglobulins, rituximab, cytotoxic therapies, danazol, and
azathioprine) and 40% of the patients had undergone splenectomy. The median
baseline platelet counts (approximately 18 x 10 9/L) were similar among all
treatment groups.
Study 773B randomized 114 patients (2:1) to eltrombopag 50 mg or placebo. Of
60 patients with documented time since diagnosis, approximately 17% met the
definition of persistent ITP with time since diagnosis of 3 to 12 months.
Study 773A randomized 117 patients (1:1:1:1) among placebo or 1 of 3 dose
regimens of eltrombopag, 30 mg, 50 mg, or 75 mg each administered daily. Of 51
patients with documented time since diagnosis, approximately 14% met the
definition of persistent ITP.
The efficacy of eltrombopag in this trial was evaluated by response rate,
defined as a shift from a baseline platelet count of less than 30 x 10 9/L to
greater than or equal to 50 x 10 9/L at any time during the treatment period
(Table 16).
Table 16. Studies 773B and 773A: Platelet Count Response (≥50 x 10
9/L) Rates in Adults With Persistent or Chronic Immune
Thrombocytopenia
|
Study |
Eltrombopag |
Placebo |
|
773B |
43/73 (59%) a |
6/37 (16%) |
|
773A |
19/27 (70%) a |
3/27 (11%) |
ap-value < 0.001 for eltrombopag versus placebo.
The platelet count response to eltrombopag was similar among patients who had
or had not undergone splenectomy. In general, increases in platelet counts
were detected 1 week following initiation of eltrombopag and the maximum
response was observed after 2 weeks of therapy. In the placebo and 50 mg–dose
groups of eltrombopag, the trial drug was discontinued due to an increase in
platelet counts to greater than 200 x 10 9/L in 3% and 27% of the patients,
respectively. The median duration of treatment with the 50 mg dose of
eltrombopag was 43 days in Study 773B and 42 days in Study 773A.
Of 7 patients who underwent hemostatic challenges, additional ITP medications
were required in 3 of 3 placebo group patients and 0 of 4 patients treated
with eltrombopag. Surgical procedures accounted for most of the hemostatic
challenges. Hemorrhage requiring transfusion occurred in one placebo group
patient and no patients treated with eltrombopag.
In the RAISE study (NCT00370331), 197 patients were randomized (2:1) to
receive either eltrombopag 50 mg once daily (n = 135) or placebo (n = 62) for
6 months, during which time the dose of eltrombopag could be adjusted based on
individual platelet counts. Of 145 patients with documented time since
diagnosis, 19% met the definition of persistent ITP. Patients were allowed to
taper or discontinue concomitant ITP medications after being treated with
eltrombopag for 6 weeks. Patients were permitted to receive rescue treatments
at any time during the trial as clinically indicated.
The median ages of the patients treated with eltrombopag and placebo were 47
years and 52.5 years, respectively. Approximately half of the patients treated
with eltrombopag and placebo (47% and 50%, respectively) were receiving
concomitant ITP medication (predominantly corticosteroids) at randomization
and had baseline platelet counts less than or equal to 15 x 10 9/L (50% and
48%, respectively). A similar percentage of patients treated with eltrombopag
and placebo (37% and 34%, respectively) had a prior splenectomy.
The efficacy of eltrombopag in this trial was evaluated by the odds of
achieving a platelet count greater than or equal to 50 x 10 9/L and less than
or equal to 400 x 10 9/L for patients receiving eltrombopag relative to
placebo and was based on patient response profiles throughout the 6-month
treatment period. In 134 patients who completed 26 weeks of treatment, a
sustained platelet response (platelet count greater than or equal to 50 x 10
9/L and less than or equal to 400 x 10 9/L for 6 out of the last 8 weeks of
the 26-week treatment period in the absence of rescue medication at any time)
was achieved by 60% of patients treated with eltrombopag, compared with 10% of
patients treated with placebo (splenectomized patients: eltrombopag 51%,
placebo 8%; non-splenectomized patients: eltrombopag 66%, placebo 11%). The
proportion of responders in the group of patients treated with eltrombopag was
between 37% and 56% compared with 7% and 19% in the placebo treatment group
for all on-therapy visits. Patients treated with eltrombopag were
significantly more likely to achieve a platelet count between 50 x 10 9/L and
400 x 10 9/L during the entire 6-month treatment period compared with those
patients treated with placebo.
Outcomes of treatment are presented in Table 17 for all patients enrolled in
the trial.
Table 17. RAISE: Outcomes of Treatment in Adults With Persistent or Chronic Immune Thrombocytopenia
|
Outcome |
Eltrombopag |
Placebo |
|
Mean number of weeks with platelet counts ≥ 50 x 10 9/L |
11.3 |
2.4 |
|
Requiring rescue therapy, n (%) |
24 (18) |
25 (40) |
Among 94 patients receiving other ITP therapy at baseline, 37 (59%) of 63
patients treated with eltrombopag and 10 (32%) of 31 patients in the placebo
group discontinued concomitant therapy at some time during the trial.
In the EXTEND study (NCT00351468), patients who completed any prior clinical
trial with eltrombopag were enrolled in an open-label, single-arm trial in
which attempts were made to decrease the dose or eliminate the need for any
concomitant ITP medications. Eltrombopag was administered to 302 patients in
EXTEND; 218 patients completed 1 year, 180 patients completed 2 years, 107
patients completed 3 years, 75 patients completed 4 years, 34 patients
completed 5 years, and 18 patients completed 6 years of therapy. The median
baseline platelet count was 19 x 10 9/L prior to administration of
eltrombopag. Median platelet counts at 1, 2, 3, 4, 5, 6, and 7 years on study
were 85 x 10 9/L, 85 x 10 9/L, 105 x 10 9/L, 64 x 10 9/L, 75 x 10 9/L, 119 x
10 9/L, and 76 x 10 9/L, respectively.
Pediatric Patients:The efficacy and safety of eltrombopag in pediatric
patients 1 year and older with persistent or chronic ITP were evaluated in two
double-blind, placebo-controlled trials. The trials differed in time since ITP
diagnosis: at least 6 months versus at least 12 months. During the trials,
doses could be increased every 2 weeks to a maximum of 75 mg once daily. The
dose of eltrombopag was reduced if the platelet count exceeded 200 x 10 9/L
and interrupted and reduced if it exceeded 400 x 10 9/L.
In the PETIT2 study (NCT01520909), patients refractory or relapsed to at least
one prior ITP therapy with a platelet count less than 30 x 10 9/L (n = 92)
were stratified by age and randomized (2:1) to eltrombopag (n = 63) or placebo
(n = 29). The starting dose for patients aged 6 to 17 years was 50 mg once
daily for those at least 27 kg and 37.5 mg once daily for those less than 27
kg, administered as oral tablets. A reduced dose of 25 mg once daily was used
for East-/Southeast-Asian patients aged 6 to 17 years regardless of weight.
The starting dose for patients aged 1 to 5 years was 1.2 mg/kg once daily (0.8
mg/kg once daily for East-/Southeast-Asian patients) administered as oral
suspension.
The 13-week, randomized, double-blind period was followed by a 24-week, open-
label period where patients from both arms were eligible to receive
eltrombopag.
The median age of the patients was 9 years and 48% were female. Approximately
62% of patients had a baseline platelet count less than or equal to 15 x 10
9/L, a characteristic that was similar between treatment arms. The percentage
of patients with at least 2 prior ITP therapies (predominantly corticosteroids
and immunoglobulins) was 73% in the group treated with eltrombopag and 90% in
the group treated with placebo. Four patients in the group treated with
eltrombopag had undergone splenectomy.
The efficacy of eltrombopag in this trial was evaluated by the proportion of
subjects on eltrombopag achieving platelet counts ≥ 50 x 10 9/L (in the
absence of rescue therapy) for at least 6 out of 8 weeks between Weeks 5 to 12
of the randomized, double-blind period (Table 18).
Table 18. PETIT2: Platelet Count Response (≥ 50 x 109/L Without
Rescue) for 6 out of 8 Weeks (between Weeks 5 to 12) Overall and by Age Cohort
in Pediatric Patients 1 Year and Older With Chronic Immune Thrombocytopenia
|
Age cohort |
Eltrombopag |
Placebo |
|
Overall |
26/63 (41%) a |
1/29 (3%) |
ap-value = < 0.001 for eltrombopag versus placebo.
More pediatric patients treated with eltrombopag (75%) compared with placebo
(21%) had at least one platelet count greater than or equal to 50 x 10 9/L
during the first 12 weeks of randomized treatment in absence of rescue
therapy. Fewer pediatric patients treated with eltrombopag required rescue
treatment during the randomized, double-blind period compared with placebo-
treated patients (19% [12/63] versus 24% [7/29]). In the patients who achieved
a platelet response (≥ 50 x 10 9/L without rescue) for 6 out of 8 weeks
(between weeks 5 to 12), 62% (16/26) had an initial response in the first 2
weeks after starting eltrombopag.
Patients were permitted to reduce or discontinue baseline ITP therapy only
during the open-label phase of the trial. Among 15 patients receiving other
ITP therapy at baseline, 53% (8/15) reduced (n = 1) or discontinued (n = 7)
concomitant therapy, mainly corticosteroids, without needing rescue therapy.
In the PETIT study (NCT00908037), patients refractory or relapsed to at least
one prior ITP therapy with a platelet count less than 30 x 10 9/L (n = 67)
were stratified by age and randomized (2:1) to eltrombopag (n = 45) or placebo
(n = 22). Approximately 15% of patients met the definition of persistent ITP.
The starting dose for patients aged 12 to 17 years was 37.5 mg once daily
regardless of weight or race. The starting dose for patients aged 6 to 11
years was 50 mg once daily for those greater than or equal to 27 kg and 25 mg
once daily for those less than 27 kg, administered as oral tablets. Reduced
doses of 25 mg (for those greater than or equal to 27 kg) and 12.5 mg (for
those less than 27 kg), each once daily, were used for East-/Southeast-Asian
patients in this age range. The starting dose for patients aged 1 to 5 years
was 1.5 mg/kg once daily (0.8 mg/kg once daily for East-/Southeast-Asian
patients) administered as oral suspension.
The 7-week, randomized, double-blind period was followed by an open-label
period of up to 24 weeks where patients from both arms were eligible to
receive eltrombopag.
The median age of the patients was 10 years and 60% were female. Approximately
51% of patients had a baseline platelet count less than or equal to 15 x 10
9/L. The percentage of patients with at least 2 prior ITP therapies
(predominantly corticosteroids and immunoglobulins) was 84% in the group
treated with eltrombopag and 86% in the group treated with placebo. Five
patients in the group treated with eltrombopag had undergone splenectomy.
The efficacy of eltrombopag in this trial was evaluated by the proportion of
patients achieving platelet counts greater than or equal to 50 x 10 9/L (in
absence of rescue therapy) at least once between Weeks 1 and 6 of the
randomized, double-blind period (Table 19). Platelet response to eltrombopag
was consistent across the age cohorts.
Table 19. PETIT: Platelet Count Response (≥ 50 x 109/L Without Rescue) Rates in Pediatric Patients 1 Year and Older With Persistent or Chronic Immune Thrombocytopenia
|
Age cohort |
Eltrombopag |
Placebo |
|
Overall |
28/45 (62%) a |
7/22 (32%) |
|
12 to 17 years |
10/16 (62%) |
0/8 (0%) |
|
6 to 11 years |
12/19 (63%) |
3/9 (33%) |
|
1 to 5 years |
6/10 (60%) |
4/5 (80%) |
ap-value = 0.011 for eltrombopag versus placebo.
Fewer pediatric patients treated with eltrombopag required rescue treatment
during the randomized, double-blind period compared with placebo-treated
patients (13% [6/45] versus 50% [11/22]).
Patients were permitted to reduce or discontinue baseline ITP therapy only
during the open-label phase of the trial. Among 13 patients receiving other
ITP therapy at baseline, 46% (6/13) reduced (n = 3) or discontinued (n = 3)
concomitant therapy, mainly corticosteroids, without needing rescue therapy.
14.2 Chronic Hepatitis C-Associated Thrombocytopenia
The efficacy and safety of eltrombopag for the treatment of thrombocytopenia
in adult patients with chronic hepatitis C were evaluated in two randomized,
double-blind, placebo-controlled trials. The ENABLE1 study (NCT00516321)
utilized peginterferon alfa-2a (PEGASYS ®) plus ribavirin for antiviral
treatment and the ENABLE2 study (NCT00529568) utilized peginterferon alfa-2b
(PEGINTRON ®) plus ribavirin. In both trials, patients with a platelet count
of less than 75 x 10 9/L were enrolled and stratified by platelet count,
screening HCV RNA, and HCV genotype. Patients were excluded if they had
evidence of decompensated liver disease with Child-Pugh score greater than 6
(class B and C), history of ascites, or hepatic encephalopathy. The median age
of the patients in both trials was 52 years, 63% were male, and 74% were
Caucasian. Sixty-nine percent of patients had HCV genotypes 1, 4, 6, with the
remainder genotypes 2 and 3. Approximately 30% of patients had been previously
treated with interferon and ribavirin. The majority of patients (90%) had
bridging fibrosis and cirrhosis, as indicated by noninvasive testing. A
similar proportion (95%) of patients in both treatment groups had Child-Pugh
class A (score 5 to 6) at baseline. A similar proportion of patients (2%) in
both treatment groups had baseline international normalized ratio (INR)
greater than 1.7. Median baseline platelet counts (approximately 60 x 10 9/L)
were similar in both treatment groups. The trials consisted of 2 phases – a
pre-antiviral treatment phase and an antiviral treatment phase. In the pre-
antiviral treatment phase, patients received open-label eltrombopag to
increase the platelet count to a threshold of greater than or equal to 90 x 10
9/L for ENABLE1 and greater than or equal to 100 x 10 9/L for ENABLE2.
Eltrombopag was administered at an initial dose of 25 mg once daily for 2
weeks and increased in 25 mg increments over 2- to 3-week periods to achieve
the optimal platelet count to initiate antiviral therapy. The maximal time
patients could receive open-label eltrombopag was 9 weeks. If threshold
platelet counts were achieved, patients were randomized (2:1) to the same dose
of eltrombopag at the end of the pre-treatment phase or to placebo.
Eltrombopag was administered in combination with pegylated interferon and
ribavirin per their respective prescribing information for up to 48 weeks.
The efficacy of eltrombopag for both trials was evaluated by sustained
virologic response (SVR) defined as the percentage of patients with
undetectable HCV-RNA at 24 weeks after completion of antiviral treatment. The
median time to achieve the target platelet count greater than or equal to 90 x
10 9/L was approximately 2 weeks. Ninety-five percent of patients were able to
initiate antiviral therapy.
In both trials, a significantly greater proportion of patients treated with
eltrombopag achieved SVR (see Table 20). The improvement in the proportion of
patients who achieved SVR was consistent across subgroups based on baseline
platelet count (less than 50 x 10 9/L versus greater than or equal to 50 x 10
9/L). In patients with high baseline viral loads (greater than or equal to
800,000), the SVR rate was 18% (82/452) for eltrombopag versus 8% (20/239) for
placebo.
Table 20. ENABLE1 and ENABLE2: Sustained Virologic Response (SVR) in Adults With Chronic Hepatitis C
|
Pre-antiviral treatment phase |
ENABLE1****a |
ENABLE2****b | ||
|
n = 715 |
n = 805 | |||
|
% Patients who achieved target platelet counts and initiated antiviral therapy c |
95% |
94% | ||
|
Antiviral treatment phase |
Eltrombopag |
Placebo |
Eltrombopag |
Placebo |
|
Overall SVR****d |
23 |
14 |
19 |
13 |
Abbreviation: HCV, hepatitis C virus.
aEltrombopag given in combination with peginterferon alfa-2a (180 mcg once
weekly for 48 weeks for genotypes 1/4/6; 24 weeks for genotype 2 or 3) plus
ribavirin (800 to 1,200 mg daily in 2 divided doses orally).
bEltrombopag given in combination with peginterferon alfa-2b (1.5 mcg/kg once
weekly for 48 weeks for genotypes 1/4/6; 24 weeks for genotype 2 or 3) plus
ribavirin (800 to 1,400 mg daily in 2 divided doses orally).
cTarget platelet count was ≥ 90 x 10 9/L for ENABLE1 and ≥ 100 x 10 9/L for
ENABLE2.
d p-value < 0.05 for eltrombopag versus placebo.
The majority of patients treated with eltrombopag (76%) maintained a platelet count greater than or equal to 50 x 10 9/L compared with 19% for placebo. A greater proportion of patients on eltrombopag did not require any antiviral dose reduction as compared with placebo (45% versus 27%).
14.3 Severe Aplastic Anemia
Refractory Severe Aplastic Anemia
Eltrombopag was studied in a single-arm, single-center, open-label trial
(Study ETB115AUS28T, referred to as Study US28T [NCT00922883]) in 43 patients
with severe aplastic anemia who had an insufficient response to at least one
prior immunosuppressive therapy and who had a platelet count less than or
equal to 30 x 10 9/L. Eltrombopag was administered at an initial dose of 50 mg
once daily for 2 weeks and increased over 2-week periods up to a maximum dose
of 150 mg once daily. The efficacy of eltrombopag in the study was evaluated
by the hematologic response assessed after 12 weeks of treatment. Hematologic
response was defined as meeting 1 or more of the following criteria: 1)
platelet count increases to 20 x 10 9/L above baseline, or stable platelet
counts with transfusion independence for a minimum of 8 weeks; 2) hemoglobin
increase by greater than 1.5 g/dL, or a reduction in greater than or equal to
4 units of red blood cell (RBC) transfusions for 8 consecutive weeks; 3) ANC
increase of 100% or an ANC increase greater than 0.5 x 10 9/L. Eltrombopag was
discontinued after 16 weeks if no hematologic response was observed. Patients
who responded continued therapy in an extension phase of the trial.
The treated population had median age of 45 years (range, 17 to 77 years) and
56% were male. At baseline, the median platelet count was 20 x 10 9/L,
hemoglobin was 8.4 g/dL, ANC was 0.58 x 10 9/L, and absolute reticulocyte
count was 24.3 x 10 9/L. Eighty-six percent of patients were red blood cell
(RBC) transfusion dependent and 91% were platelet transfusion dependent. The
majority of patients (84%) received at least 2 prior immunosuppressive
therapies. Three patients had cytogenetic abnormalities at baseline.
Table 23 presents the efficacy results.
Table 23. Study US28T: Hematologic Response in Patients With Refractory
Severe Aplastic Anemia
|
Outcome |
Eltrombopag |
|
Response rate a, n (%) |
17 (40) |
|
Median of duration of response in months (95% CI) |
NR b(3.0, NR b) |
aIncludes single-and multi-lineage.
bNR = not reached due to few events (relapsed).
In the 17 responders, the platelet transfusion-free period ranged from 8 to
1096 days with a median of 200 days, and the RBC transfusion-free period
ranged from 15 to 1082 days with a median of 208 days.
In the extension phase, 8 patients achieved a multi-lineage response; 4 of
these patients subsequently tapered off treatment with eltrombopag and
maintained the response (median follow-up: 8.1 months,
range, 7.2 to 10.6 months).
Additional pediatric use information is approved for Novartis Pharmaceuticals
Corporation's PROMACTA ®(eltrombopag) tablets. However, due to Novartis
Pharmaceuticals Corporation's marketing exclusivity rights, this drug product
is not labeled with that information.
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Persistent or Chronic Immune Thrombocytopenia
Use the lowest dose of eltrombopag tablets to achieve and maintain a platelet
count greater than or equal to 50 x 10 9/L as necessary to reduce the risk for
bleeding. Dose adjustments are based upon the platelet count response. Do not
use eltrombopag tablets to normalize platelet counts [see Warnings and Precautions (5.4)] . In clinical trials, platelet counts generally increased
within 1 to 2 weeks after starting eltrombopag tablets and decreased within 1
to 2 weeks after discontinuing eltrombopag tablets [see Clinical Studies (14.1)].
Initial Dose Regimen:
Adult and Pediatric Patients 6 Years and Older with ITP:Initiate eltrombopag
tablets at a dose of 50 mg orally once daily, except in patients who are of
East-/Southeast-Asian ancestry or who have mild to severe hepatic impairment
(Child-Pugh class A, B, C).
For patients of East-/Southeast-Asian ancestry with ITP, initiate eltrombopag
tablets at a reduced dose of 25 mg orally once daily [see Use in Specific Populations (8.7), Clinical Pharmacology (12.3)].
For patients with ITP and mild, moderate, or severe hepatic impairment (Child-
Pugh class A, B, C), initiate eltrombopag tablets at a reduced dose of 25 mg
orally once daily [see Use in Specific Populations (8.6), Clinical Pharmacology (12.3)].
For patients of East-/Southeast-Asian ancestry with ITP and hepatic impairment
(Child-Pugh class A, B, C), consider initiating eltrombopag tablets at a
reduced dose of 12.5 mg orally once daily [see Clinical Pharmacology (12.3)].
Pediatric Patients with ITP Aged 1 to 5 Years:Initiate eltrombopag tablets at
a dose of 25 mg orally once daily [see Use in Specific Populations (8.7), Clinical Pharmacology (12.3)].
Monitoring and Dose Adjustment:After initiating eltrombopag tablets, adjust
the dose to achieve and maintain a platelet count greater than or equal to 50
x 10 9/L as necessary to reduce the risk for bleeding. Do not exceed a dose of
75 mg daily. Monitor clinical hematology and liver tests regularly throughout
therapy with eltrombopag tablets and modify the dosage regimen of eltrombopag
tablets based on platelet counts as outlined in Table 1. During therapy with
eltrombopag tablets, assess complete blood counts (CBCs) with differentials,
including platelet counts, weekly until a stable platelet count has been
achieved. Obtain CBCs with differentials, including platelet counts, monthly
thereafter.
When switching between the oral suspension and tablet, assess platelet counts
weekly for 2 weeks, and then follow standard monthly monitoring.
Table 1. Dose Adjustments of Eltrombopag Tablets in Patients With Persistent or Chronic Immune Thrombocytopenia
|
Platelet count result |
Dose adjustment or response |
|
< 50 x 10 9/L following at least 2 weeks of eltrombopag tablets |
Increase daily dose by 25 mg to a maximum of 75 mg/day. For patients taking 12.5 mg once daily, increase the dose to 25 mg daily before increasing the dose amount by 25 mg. |
|
≥ 200 x 10 9/L to ≤ 400 x 10 9/L at any time |
Decrease the daily dose by 25 mg. Wait 2 weeks to assess the effects of this and any subsequent dose adjustments. For patients taking 25 mg once daily, decrease the dose to 12.5 mg once daily. |
|
Stop eltrombopag tablets; increase the frequency of platelet monitoring to twice weekly. Once the platelet count is < 150 x 10 9/L, reinitiate therapy at a daily dose reduced by 25 mg. For patients taking 25 mg once daily, reinitiate therapy at a daily dose of 12.5 mg. |
|
Discontinue eltrombopag tablets. |
In patients with ITP and hepatic impairment (Child-Pugh class A, B, C), after
initiating eltrombopag tablets or after any subsequent dosing increase, wait 3
weeks before increasing the dose.
Modify the dosage regimen of concomitant ITP medications, as medically
appropriate, to avoid excessive increases in platelet counts during therapy
with eltrombopag tablets. Do not administer more than one dose of eltrombopag
tablets within any 24-hour period.
Discontinuation:Discontinue eltrombopag tablets if the platelet count does not
increase to a level sufficient to avoid clinically important bleeding after 4
weeks of therapy with eltrombopag tablets at the maximum daily dose of 75 mg.
Excessive platelet count responses, as outlined in Table 1, or important liver
test abnormalities (e.g., transaminases and/or bilirubin) also necessitate
discontinuation of eltrombopag tablets [see Warnings and Precautions (5.2, 5.6) and Drug Interactions (7.5)]. Obtain CBCs with differentials, including
platelet counts, weekly for at least 4 weeks following discontinuation of
eltrombopag tablets.
2.2 Chronic Hepatitis C-Associated Thrombocytopenia
Use the lowest dose of eltrombopag tablets to achieve and maintain a platelet
count necessary to initiate and maintain antiviral therapy with pegylated
interferon and ribavirin. Dose adjustments are based upon the platelet count
response. Do not use eltrombopag tablets to normalize platelet counts [see Warnings and Precautions (5.4)] . In clinical trials, platelet counts
generally began to rise within the first week of treatment with eltrombopag
tablets [see Clinical Studies (14.2)].
Initial Dose Regimen:Initiate eltrombopag tablets at a dose of 25 mg orally
once daily.
Monitoring and Dose Adjustment:Adjust the dose of eltrombopag tablets in 25 mg
increments every 2 weeks as necessary to achieve the target platelet count
required to initiate antiviral therapy. Monitor platelet counts every week
prior to starting antiviral therapy.
During antiviral therapy, adjust the dose of eltrombopag tablets to avoid dose
reductions of peginterferon. Monitor CBCs with differentials, including
platelet counts, weekly during antiviral therapy until a stable platelet count
is achieved. Monitor platelet counts monthly thereafter. Do not exceed a dose
of 100 mg daily. Monitor clinical hematology and liver tests (e.g.,
transaminases and bilirubin) regularly throughout therapy with eltrombopag
tablets [see Drug Interactions (7.5)].
For specific dosage instructions for peginterferon or ribavirin, refer to
their respective prescribing information.
Table 2. Dose Adjustments of Eltrombopag Tablets in Adults With Thrombocytopenia Due to Chronic Hepatitis C
|
Platelet count result |
Dose adjustment or response |
|
< 50 x 10 9/L following at least 2 weeks of eltrombopag tablets |
Increase daily dose by 25 mg to a maximum of 100 mg/day. |
|
≥ 200 x 10 9/L to ≤ 400 x 10 9/L at any time |
Decrease the daily dose by 25 mg. Wait 2 weeks to assess the effects of this and any subsequent dose adjustments. |
|
Stop eltrombopag tablets; increase the frequency of platelet monitoring to twice weekly. Once the platelet count is < 150 x 10 9/L, reinitiate therapy at a daily dose reduced by 25 mg. For patients taking 25 mg once daily, reinitiate therapy at a daily dose of 12.5 mg. |
|
Discontinue eltrombopag tablets. |
Discontinuation:The prescribing information for pegylated interferon and
ribavirin include recommendations for antiviral treatment discontinuation for
treatment futility. Refer to pegylated interferon and ribavirin prescribing
information for discontinuation recommendations for antiviral treatment
futility.
Eltrombopag tablets should be discontinued when antiviral therapy is
discontinued. Excessive platelet count responses, as outlined in Table 2, or
important liver test abnormalities also necessitate discontinuation of
eltrombopag tablets [see Warnings and Precautions (5.2)].
2.3 Severe Aplastic Anemia
Refractory Severe Aplastic Anemia
Use the lowest dose of eltrombopag tablets to achieve and maintain a
hematologic response. Dose adjustments are based upon the platelet count.
Hematologic response requires dose titration, generally up to 150 mg, and may
take up to 16 weeks after starting eltrombopag tablets [see Clinical Studies (14.3)].
Initial Dose Regimen:Initiate eltrombopag tablets at a dose of 50 mg orally
once daily.
For patients with severe aplastic anemia of East-/Southeast-Asian ancestry or
those with mild, moderate, or severe hepatic impairment (Child-Pugh class A,
B, C), initiate eltrombopag tablets at a reduced dose of 25 mg orally once
daily [see Use in Specific Populations (8.6, 8.7), Clinical Pharmacology (12.3)].
Monitoring and Dose Adjustment:Adjust the dose of eltrombopag tablets in 50 mg
increments every 2 weeks as necessary to achieve the target platelet count
greater than or equal to 50 x 10 9/L as necessary. Do not exceed a dose of 150
mg daily. Monitor clinical hematology and liver tests regularly throughout
therapy with eltrombopag tablets and modify the dosage regimen of eltrombopag
tablets based on platelet counts as outlined in Table 7.
Table 7. Dose Adjustments of Eltrombopag Tablets in Patients With Refractory Severe Aplastic Anemia
|
Platelet count result |
Dose adjustment or response |
|
< 50 x 10 9/L following at least 2 weeks of eltrombopag tablets |
Increase daily dose by 50 mg to a maximum of 150 mg/day. For patients taking 25 mg once daily, increase the dose to 50 mg daily before increasing the dose amount by 50 mg. |
|
≥ 200 x 10 9/L to ≤ 400 x 10 9/L at any time |
Decrease the daily dose by 50 mg. Wait 2 weeks to assess the effects of this and any subsequent dose adjustments. |
|
Stop eltrombopag tablets for 1 week. Once the platelet count is < 150 x 10 9/L, reinitiate therapy at a dose reduced by 50 mg. |
|
Discontinue eltrombopag tablets. |
For patients who achieve tri-lineage response, including transfusion
independence, lasting at least 8 weeks: the dose of eltrombopag tablets may be
reduced by 50% [see Clinical Studies (14.3)]. If counts remain stable after 8
weeks at the reduced dose, then discontinue eltrombopag tablets and monitor
blood counts. If platelet counts drop to less than 30 x 10 9/L, hemoglobin to
less than 9 g/dL, or absolute neutrophil count (ANC) to less than 0.5 x 10
9/L, eltrombopag tablets may be reinitiated at the previous effective dose.
Discontinuation:If no hematologic response has occurred after 16 weeks of
therapy with eltrombopag tablets, discontinue therapy. If new cytogenetic
abnormalities are observed, consider discontinuation of eltrombopag tablets
[see Adverse Reactions (6.1)]. Excessive platelet count
responses (as outlined in Table 7) or important liver test abnormalities also
necessitate discontinuation of eltrombopag tablets [see Warnings and Precautions (5.2)].
2.4 Administration
Administration of Tablets:Take eltrombopag tablets without a meal or with a
meal low in calcium (≤ 50 mg). Take eltrombopag tablets at least 2 hours
before or 4 hours after other medications (e.g., antacids), calcium-rich foods
(containing > 50 mg calcium e.g., dairy products, calcium-fortified juices,
and certain fruits and vegetables), or supplements containing polyvalent
cations, such as iron, calcium, aluminum, magnesium, selenium, and zinc [see Drug Interactions (7.1), Clinical Pharmacology (12.3)].
Do not split, chew, or crush tablets and mix with food or liquids.
Additional pediatric use information is approved for Novartis Pharmaceuticals
Corporation's PROMACTA ®(eltrombopag) tablets. However, due to Novartis
Pharmaceuticals Corporation's marketing exclusivity rights, this drug product
is not labeled with that information.
• Take eltrombopag tablets without a meal or with a meal low in calcium (≤ 50 mg). Take eltrombopag tablets at least 2 hours before or 4 hours after any medications or products containing polyvalent cations, such as antacids, calcium-rich foods, and mineral supplements. (2.4, 7.1, 12.3)
• Persistent or Chronic ITP:Initiate eltrombopag tablets at 50 mg orally once daily for most adult and pediatric patients 6 years and older, and at 25 mg orally once daily for pediatric patients aged 1 to 5 years. Dose reductions are needed for patients with hepatic impairment and some patients of East-/Southeast-Asian ancestry. Adjust to maintain platelet count greater than or equal to 50 x 10 9/L. Do not exceed 75 mg per day. (2.1, 8.6, 8.7)
• Chronic Hepatitis C-associated Thrombocytopenia:Initiate eltrombopag tablets
at 25 mg orally once daily for all patients. Adjust to achieve target platelet
count required to initiate antiviral therapy. Do not exceed a daily dose of
100 mg. (2.2)
• Refractory Severe Aplastic Anemia:Initiate eltrombopag tablets at 50 mg
orally once daily. Reduce initial dose in patients with hepatic impairment or
patients of East-/Southeast-Asian ancestry. Adjust to maintain platelet count
greater than 50 x 10 9/L. Do not exceed 150 mg per day. (2.3, 8.6, 8.7)
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
Tablets
• 12.5 mg tablets — Off-white, round, bevel edged, biconvex film-coated
tablets debossed with 'H' on one side and 'E10' on the other side. Each
tablet, for oral administration, contains eltrombopag olamine, equivalent to
12.5 mg of eltrombopag free acid.
• 25 mg tablets — Beige colored, round, bevel edged, biconvex film-coated
tablets debossed with 'H' on one side and 'E11' on the other side. Each
tablet, for oral administration, contains eltrombopag olamine, equivalent to
25 mg of eltrombopag free acid.
• 50 mg tablets — Off-white, round, bevel edged, biconvex film-coated tablets
debossed with 'H' on one side and 'E12' on the other side. Each tablet, for
oral administration, contains eltrombopag olamine, equivalent to 50 mg of
eltrombopag free acid.
• 75 mg tablets — Off-white to light yellow colored, round, bevel edged,
biconvex film-coated tablets debossed with 'H' on one side and 'E13' on the
other side. Each tablet, for oral administration, contains eltrombopag
olamine, equivalent to 75 mg of eltrombopag free acid.
- Tablets: 12.5 mg, 25 mg, 50 mg, and 75 mg (3)
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk SummaryAvailable data from a small number of published case reports and
postmarketing experience with eltrombopag use in pregnant women are
insufficient to assess any drug-associated risks for major birth defects,
miscarriage, or adverse maternal or fetal outcomes. In animal reproduction and
developmental toxicity studies, oral administration of eltrombopag to pregnant
rats during organogenesis resulted in embryolethality and reduced fetal
weights at maternally toxic doses. These effects were observed at doses
resulting in exposures that were six times the human clinical exposure based
on area under the curve (AUC) in patients with persistent or chronic ITP at 75
mg/day, and three times the AUC in patients with chronic hepatitis C at 100
mg/day (see Data).
The estimated background risk of major birth defects and miscarriage for the
indicated population is unknown. All pregnancies have a background risk of
birth defect, loss, or other adverse outcomes. In the U.S. general population,
the estimated background risk of major birth defects and of miscarriage in
clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
In an early embryonic development study, female rats received oral eltrombopag
at doses of 10, 20, or 60 mg/kg/day (0.8, 2, and 6 times, respectively, the
human clinical exposure based on AUC in patients with ITP at 75 mg/day and
0.3, 1, and 3 times, respectively, the human clinical exposure based on AUC in
patients with chronic hepatitis C at 100 mg/day). Increased pre-and post-
implantation loss and reduced fetal weight were observed at the highest dose
which also caused maternal toxicity.
In an embryo-fetal development study eltrombopag was administered orally to
pregnant rats during the period of organogenesis at doses of 10, 20, or 60
mg/kg/day (0.8, 2, and 6 times, respectively, the human clinical exposure
based on AUC in patients with ITP at 75 mg/day and 0.3, 1, and 3 times,
respectively, the human clinical exposure based on AUC in patients with
chronic hepatitis C at 100 mg/day). Decreased fetal weights (6% to 7%) and a
slight increase in the presence of cervical ribs were observed at the highest
dose which also caused maternal toxicity. However, no evidence of major
structural malformations was observed.
In an embryo-fetal development study eltrombopag was administered orally to
pregnant rabbits during the period of organogenesis at doses of 30, 80, or 150
mg/kg/day (0.04, 0.3, and 0.5 times, respectively, the human clinical exposure
based on AUC in patients with ITP at 75 mg/day and 0.02, 0.1, and 0.3 times,
respectively, the human clinical exposure based on AUC in patients with
chronic hepatitis C at 100 mg/day). No evidence of fetotoxicity,
embryolethality, or teratogenicity was observed.
In a pre-and post-natal developmental toxicity study in pregnant rats (F0),
oral eltrombopag was administered from gestation Day 6 through lactation Day
20. No adverse effects on maternal reproductive function or on the development
of the offspring (F1) were observed at doses up to 20 mg/kg/day (2 times the
human clinical exposure based on AUC in patients with ITP at 75 mg/day and
similar to the human clinical exposure based on AUC in patients with chronic
hepatitis C at 100 mg/day). Eltrombopag was detected in the plasma of
offspring (F1). The plasma concentrations in pups increased with dose
following administration of drug to the F0 dams.
8.2 Lactation
Risk Summary
There are no data regarding the presence of eltrombopag or its metabolites in
human milk, the effects on the breastfed child, or the effects on milk
production. However, eltrombopag was detected in the pups of lactating rats 10
days postpartum suggesting the potential for transfer during lactation. Due to
the potential for serious adverse reactions in a breastfed child from
eltrombopag, breastfeeding is not recommended during treatment.
8.3 Females and Males of Reproductive Potential
Contraception
Based on animal reproduction studies, eltrombopag can cause fetal harm when
administered to a pregnant woman. Sexually-active females of reproductive
potential should use effective contraception (methods that result in less than
1% pregnancy rates) when using eltrombopag during treatment and for at least 7
days after stopping treatment with eltrombopag.
8.4 Pediatric Use
The safety and efficacy of eltrombopag have been established in pediatric
patients 1 year and older with persistent or chronic ITP. Safety and efficacy
in pediatric patients below the age of 1 year with ITP have not been
established. Safety and efficacy in pediatric patients with thrombocytopenia
associated with chronic hepatitis C and refractory severe aplastic anemia have
not been established.
The safety and efficacy of eltrombopag in pediatric patients 1 year and older
with persistent or chronic ITP were evaluated in two double-blind, placebo-
controlled trials [see Adverse Reactions (6.1), Clinical Studies (14.1)] . The
pharmacokinetics of eltrombopag have been evaluated in 168 pediatric patients
1 year and older with ITP dosed once daily [see Clinical Pharmacology (12.3)]
. See Dosage and Administration (2.1)for dosing recommendations for pediatric
patients 1 year and older.
Additional pediatric use information is approved for Novartis Pharmaceuticals
Corporation's PROMACTA ®(eltrombopag) tablets. However, due to Novartis
Pharmaceuticals Corporation's marketing exclusivity rights, this drug product
is not labeled with that information.
8.5 Geriatric Use
Of the 106 patients in two randomized clinical trials of eltrombopag 50 mg in persistent or chronic ITP, 22% were 65 years of age and over, while 9% were 75 years of age and over. Of the 1439 patients in two randomized clinical trials of eltrombopag in patients with chronic hepatitis C and thrombocytopenia, 7% were 65 years of age and over, while < 1% were 75 years of age and over. Of the 196 patients who received eltrombopag for the treatment of severe aplastic anemia, 18% were 65 years of age and over, while 3% were 75 years of age and over. No overall differences in safety or effectiveness were observed between these patients and younger patients.
8.6 Hepatic Impairment
Patients With Persistent or Chronic ITP and Severe Aplastic Anemia
Reduce the initial dose of eltrombopag in patients with persistent or chronic
ITP (adult and pediatric patients 6 years and older only) or refractory severe
aplastic anemia who also have hepatic impairment (Child-Pugh class A, B, C)
[see Dosage and Administration (2.1, 2.3), Warnings and Precautions (5.2), Clinical Pharmacology (12.3)].
Patients With Chronic Hepatitis C
No dosage adjustment is recommended in patients with chronic hepatitis C and
hepatic impairment [see Clinical Pharmacology (12.3)].
8.7 Ethnicity
Reduce the initial dose of eltrombopag for patients of East-/Southeast-Asian
ancestry with ITP (adult and pediatric patients 6 years and older only) or
severe aplastic anemia [see Dosage and Administration (2.1, 2.3), Clinical Pharmacology (12.3)]. No reduction in the initial dose of eltrombopag is
recommended in patients of East-/Southeast-Asian ancestry with chronic
hepatitis C [see Clinical Pharmacology (12.3)].
Additional pediatric use information is approved for Novartis Pharmaceuticals
Corporation's PROMACTA ®(eltrombopag) tablets. However, due to Novartis
Pharmaceuticals Corporation's marketing exclusivity rights, this drug product
is not labeled with that information.
• Lactation:Advise women not to breastfeed during treatment. (8.2)
Additional pediatric use information is approved for Novartis Pharmaceuticals
Corporation's PROMACTA ®(eltrombopag) tablets. However, due to Novartis
Pharmaceuticals Corporation's marketing exclusivity rights, this drug product
is not labeled with that information.
OVERDOSAGE SECTION
10 OVERDOSAGE
In the event of overdose, platelet counts may increase excessively and result
in thrombotic/thromboembolic complications.
In one report, a subject who ingested 5000 mg of eltrombopag had a platelet
count increase to a maximum of 929 x 10 9/L at 13 days following the
ingestion. The patient also experienced rash, bradycardia, ALT/AST elevations,
and fatigue. The patient was treated with gastric lavage, oral lactulose,
intravenous fluids, omeprazole, atropine, furosemide, calcium, dexamethasone,
and plasmapheresis; however, the abnormal platelet count and liver test
abnormalities persisted for 3 weeks. After 2 months’ follow-up, all events had
resolved without sequelae.
In case of an overdose, consider oral administration of a metal cation-
containing preparation, such as calcium, aluminum, or magnesium preparations
to chelate eltrombopag and thus limit absorption. Closely monitor platelet
counts. Reinitiate treatment with eltrombopag in accordance with dosing and
administration recommendations [see Dosage and Administration (2.1, 2.2)].
Consider contacting the Poison Help line (1-800-222-1222) or a medical
toxicologist for additional overdose management recommendations.
DESCRIPTION SECTION
11 DESCRIPTION
Eltrombopag tablets contain eltrombopag olamine, a small molecule
thrombopoietin (TPO) receptor agonist for oral administration.
Eltrombopag olamine is a biphenyl hydrazone. The chemical name for eltrombopag
olamine is
3'-{(2Z)-2-[1-(3,4-dimethylphenyl)-3-methyl-5-oxo-1,5-dihydro-4H-pyrazol-4ylidene]hydrazino}-2'-hydroxy-3-biphenylcarboxylic
acid, ethanolamine. It has the molecular formula C 25H 22N 4O 4.C 4H 14N 2O 2.
The molecular weight is 564.27 g/mol for eltrombopag olamine and 442.5 g/mol
for eltrombopag free acid. Eltrombopag olamine has the following structural
formula:
Eltrombopag olamine is very slightly soluble in methanol and dimethyl
formamide.
Eltrombopag tablets contain eltrombopag olamine in the amount equivalent to
12.5 mg, 25 mg, 50 mg, or 75 mg of eltrombopag free acid. The inactive
ingredients of eltrombopag tablets are:
**Tablet Core:**magnesium stearate, mannitol, microcrystalline cellulose,
povidone and sodium starch glycolate.
**Coating:**FD&C Blue #2/Indigo carmine aluminum lake (for 25 mg), FD & C
Yellow # 6/Sunset Yellow FCF Aluminum lake (for 25 mg), hypromellose, iron
oxide yellow (for 75 mg), polyethylene glycol, polysorbate 80 (for 12.5 mg and
75 mg) and titanium dioxide.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Eltrombopag does not stimulate platelet production in rats, mice, or dogs
because of unique TPO receptor specificity. Data from these animals do not
fully model effects in humans.
Eltrombopag was not carcinogenic in mice at doses up to 75 mg/kg/day or in
rats at doses up to 40 mg/kg/day (exposures up to 4 times the human clinical
exposure based on AUC in patients with ITP at 75 mg/day and 2 times the human
clinical exposure based on AUC in patients with chronic hepatitis C at 100
mg/day).
Eltrombopag was not mutagenic or clastogenic in a bacterial mutation assay or
in two in vivoassays in rats (micronucleus and unscheduled DNA synthesis, 10
times the human clinical exposure based on C maxin patients with ITP at 75
mg/day and 7 times the human clinical exposure based on C maxin patients with
chronic hepatitis C at 100 mg/day). In the in vitromouse lymphoma assay,
eltrombopag was marginally positive (less than 3-fold increase in mutation
frequency).
Eltrombopag did not affect female fertility in rats at doses up to 20
mg/kg/day (2 times the human clinical exposure based on AUC in patients with
ITP at 75 mg/day and similar to the human clinical exposure based on AUC in
patients with chronic hepatitis C at 100 mg/day). Eltrombopag did not affect
male fertility in rats at doses up to 40 mg/kg/day, the highest dose tested (3
times the human clinical exposure based on AUC in patients with ITP at 75
mg/day and 2 times the human clinical exposure based on AUC in patients with
chronic hepatitis C at 100 mg/day).
13.2 Animal Pharmacology and/or Toxicology
Treatment-related cataracts were detected in rodents in a dose-and time-
dependent manner. At greater than or equal to 6 times the human clinical
exposure based on AUC in patients with ITP at 75 mg/day and 3 times the human
clinical exposure based on AUC in patients with chronic hepatitis C at 100
mg/day, cataracts were observed in mice after 6 weeks and in rats after 28
weeks of dosing. At greater than or equal to 4 times the human clinical
exposure based on AUC in patients with ITP at 75 mg/day and 2 times the human
clinical exposure based on AUC in patients with chronic hepatitis C at 100
mg/day, cataracts were observed in mice after 13 weeks and in rats after 39
weeks of dosing [see Warnings and Precautions (5.5)].
Renal tubular toxicity was observed in studies up to 14 days in duration in
mice and rats at exposures that were generally associated with morbidity and
mortality. Tubular toxicity was also observed in a 2-year oral carcinogenicity
study in mice at doses of 25, 75, and 150 mg/kg/day. The exposure at the
lowest dose was 1.2 times the human clinical exposure based on AUC in patients
with ITP at 75 mg/day and 0.6 times the human clinical exposure based on AUC
in patients with chronic hepatitis C at 100 mg/day. No similar effects were
observed in mice after 13 weeks at exposures greater than those associated
with renal changes in the 2-year study, suggesting that this effect is both
dose-and time-dependent.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 Tablets
• The 12.5 mg tablets are off-white, round, bevel edged, biconvex film-coated
tablets debossed with 'H' on one side and 'E10' on the other side and
are available in
Bottle of 30 tablets NDC 31722-841-30
• The 25 mg tablets are beige colored, round, bevel edged, biconvex film-
coated tablets debossed with 'H' on one side and 'E11' on the other side
and are available in
Bottle of 30 tablets NDC 31722-842-30
• The 50 mg tablets are off-white, round, bevel edged, biconvex film-coated
tablets debossed with 'H' on one side and 'E12' on the other side and
are available in
Bottle of 14 tablets NDC 31722-843-14
Bottle of 30 tablets NDC 31722-843-30
• The 75 mg tablets are off-white to light yellow colored, round, bevel edged,
biconvex film-coated tablets debossed with 'H' on one side and 'E13'
on the other side and are available in
Bottle of 30 tablets NDC 31722-844-30
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Dispense in original bottle.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient or caregiver to read the FDA-approved patient labeling
(Medication Guide).
Prior to treatment, patients should fully understand and be informed of the
following risks and considerations for eltrombopag tablets:
Risks
Hepatotoxicity
• Therapy with eltrombopag tablets may be associated with hepatobiliary
laboratory abnormalities [see Warnings and Precautions (5.2)].
• Advise patients with chronic hepatitis C and cirrhosis that they may be at
risk for hepatic decompensation when receiving eltrombopag tablets with alfa
interferon therapy [see Warnings and Precautions (5.1)].
• Advise patients that they should report any of the following signs and
symptoms of liver problems to their healthcare provider right away [see Warnings and Precautions (5.2)].
• yellowing of the skin or the whites of the eyes (jaundice)
• unusual darkening of the urine
• unusual tiredness
• right upper stomach area pain
• confusion
• swelling of the stomach area (abdomen)
Risk of Bleeding Upon Eltrombopag tablets Discontinuation
• Advise patients that thrombocytopenia and risk of bleeding may reoccur upon
discontinuing eltrombopag tablets, particularly if eltrombopag tablets are
discontinued while the patient is on anticoagulants or antiplatelet agents.
Advise patients that during therapy with eltrombopag tablets, they should
continue to avoid situations or medications that may increase the risk for
bleeding.
Thrombotic/Thromboembolic Complications
• Advise patients that too much eltrombopag tablets may result in excessive
platelet counts and a risk for thrombotic/thromboembolic complications [see Warnings and Precautions (5.4)].
Cataracts
• Advise patients to have a baseline ocular examination prior to
administration of eltrombopag tablets and be monitored for signs and symptoms
of cataracts during therapy [see Warnings and Precautions (5.5)].
Drug Interactions
• Advise patients to take eltrombopag tablets at least 2 hours before or 4
hours after calcium-rich foods, mineral supplements, and antacids which
contain polyvalent cations, such as iron, calcium, aluminum, magnesium,
selenium, and zinc [see Dosage and Administration (2.4), Drug Interactions (7.1)].
Lactation
• Advise women not to breastfeed during treatment with eltrombopag tablets
[see Use in Specific Populations (8.2)].
Administration of Eltrombopag Tablets
• For patients with persistent or chronic ITP, therapy with eltrombopag
tablets are administered to achieve and maintain a platelet count greater than
or equal to 50 x 10 9/L as necessary to reduce the risk for bleeding [see Indications and Usage (1.1)].
• For patients with chronic hepatitis C, therapy with eltrombopag tablets are
administered to achieve and maintain a platelet count necessary to initiate
and maintain antiviral therapy with pegylated interferon and ribavirin [see Indications and Usage (1.2)].
• Advise patients to take eltrombopag tablets without a meal or with a meal
low in calcium (≤ 50 mg) and at least 2 hours before or 4 hours after
other medications (e.g., antacids) and calcium-rich foods [see Dosage and Administration (2.4)].
The brands listed are the registered trademarks of their respective owners and are not trademarks of Annora Pharma Private Limited.

Manufactured for:
Camber Pharmaceuticals, Inc.
Piscataway, NJ 08854.
By: Annora Pharma Pvt. Ltd.
Sangareddy - 502313,
Telangana, India.
****(or)
By:HETERO****TM
Hetero Labs Limited, Plot No. 28P1 to 36P1 & 37 to 54,
Vemagal Industrial Area, Hobli Vemagal,
Kolar, Karnataka - 563102, India.
(or)
By: Evaric Pharmaceuticals Inc.
155 Commerce Drive,
Hauppauge, New York 11788,
United States (USA).
Revised: 08/2025
RECENT MAJOR CHANGES SECTION
RECENT MAJOR CHANGES
Warnings and Precautions, Laboratory Test Interference (5.6) 6/2025
