Exelon
Exelon
5d7c4d41-2df4-42d4-9a3d-0a567519bf2d
HUMAN PRESCRIPTION DRUG LABEL
Dec 20, 2010
Rebel Distributors Corp
DUNS: 118802834
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
rivastigmine
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (2)
Drug Labeling Information
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.1.1
Pregnancy Category B
There are no adequate or well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, Exelon Patch should be used during pregnancy only if the potential benefit outweighs the potential risk to the fetus. No dermal reproduction studies in animals have been conducted. Oral reproduction studies conducted in pregnant rats at doses up to 2.3 mg base/kg/day and in pregnant rabbits at doses up to 2.3 mg base/kg/day revealed no evidence of teratogenicity. Studies in rats showed slightly decreased fetal/pup weights, usually at doses causing some maternal toxicity.
8.
3
Nursing Mothers
Milk transfer studies in animals have not been conducted with dermal rivastigmine. In rats given rivastigmine orally, concentrations of rivastigmine plus metabolites were approximately two times higher in milk than in plasma. It is not known whether rivastigmine is excreted in human breast milk. Exelon Patch (rivastigmine transdermal system) has no indication for use in nursing mothers.
8.
4
Pediatric Use
There are no adequate and well-controlled trials documenting the safety and efficacy of Exelon in any illness occurring in children.
8.
5
Geriatric Use
Age had no impact on the exposure to rivastigmine in Alzheimer’s disease patients treated with Exelon Patch.
8.
6
Hepatic Disease
No pharmacokinetic study was conducted with Exelon Patch in subjects with hepatic impairment. Following a single 3-mg dose, mean oral clearance of rivastigmine was 60% lower in hepatically impaired patients (n=10, biopsy proven) than in healthy subjects (n=10). After multiple 6-mg BID oral dosing, the mean clearance of rivastigmine was 65% lower in mild (n=7, Child-Pugh score 5-6) and moderate (n=3, Child-Pugh score 7-9) hepatically impaired patients (biopsy proven, liver cirrhosis) than in healthy subjects (n=10). Dosage adjustment is not necessary in hepatically impaired patients as the dose of drug is individually titrated to tolerability.
8.
7
Renal Disease
No study was conducted with Exelon Patch in subjects with renal impairment. Following a single 3-mg dose, mean oral clearance of rivastigmine is 64% lower in moderately impaired renal patients (n=8, GFR=10-50 mL/min) than in healthy subjects (n=10, GFR greater than or equal to 60 mL/min); Cl/F=1.7 L/min (cv=45%) and 4.8 L/min (cv=80%), respectively. In severely impaired renal patients (n=8, GFR less than 10 mL/min), mean oral clearance of rivastigmine is 43% higher than in healthy subjects (n=10, GFR greater than or equal to 60 mL/min); Cl/F=6.9 L/min and 4.8 L/min, respectively. For unexplained reasons, the severely impaired renal patients had a higher clearance of rivastigmine than moderately impaired patients. However, dosage adjustment may not be necessary in renally impaired patients as the dose of the drug is individually titrated to tolerability.
8.
8
Low Body Weight
Rivastigmine exposure is higher in subjects with low body weight. Compared to a patient with a body weight of 65 kg, the rivastigmine steady-state concentrations in a patient with a body weight of 35 kg would be approximately doubled, while for a patient with a body weight of 100 kg the concentrations would be approximately halved. This suggests special attention should be given to patients with very low body weight during up-titration [see Dosage and Administration (2)].
8.
9
Gender and Race
No specific pharmacokinetic study was conducted to investigate the effect of gender and race on the disposition of Exelon, but a population pharmacokinetic analysis indicates that gender (n=277 males and 348 females) and race (n=575 White, 34 Black, 4 Asian, and 12 Other) did not affect the clearance of Exelon administered orally. Similar results were seen with analyses of pharmacokinetic data obtained after the administration of Exelon Patch.
8.
10
Nicotine Use
Population pharmacokinetic analysis showed that nicotine use increases the oral clearance of rivastigmine by 23% (n=75 Smokers and 549 Nonsmokers). No dose adjustment is necessary as the dose of the drug is individually titrated to tolerability.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Pathological changes in dementia of the Alzheimer’s type and dementia associated with Parkinson’s disease involve cholinergic neuronal pathways that project from the basal forebrain to the cerebral cortex and hippocampus. These pathways are thought to be intricately involved in memory, attention, learning, and other cognitive processes. While the precise mechanism of action for rivastigmine is unknown, it is postulated to exert its therapeutic effect by enhancing cholinergic function. This is accomplished by increasing the concentration of acetylcholine through reversible inhibition of its hydrolysis by cholinesterase. If this proposed mechanism is correct, the effect of rivastigmine may lessen as the disease process advances and fewer cholinergic neurons remain functionally intact. There is no evidence that rivastigmine alters the course of the underlying dementing process.
12.2 Pharmacodynamics
After a 6-mg oral dose of rivastigmine in humans, anticholinesterase activity is present in CSF for about 10 hours, with a maximum inhibition of about 60% 5 hours after dosing.
In-vitro and in-vivo studies demonstrate that the inhibition of cholinesterase by rivastigmine is not affected by the concomitant administration of memantine, an N-methyl-D-aspartate receptor antagonist.
12.3 Pharmacokinetics
12.3.1
Absorption
After the first dose, there is a lag time of 0.5-1 hour in the absorption of
rivastigmine from Exelon Patch (rivastigmine transdermal system).
Concentrations then rise slowly typically reaching a maximum after 8 hours,
although maximum values (Cmax) are often reached at later times as well (10-16
hours). After the peak, plasma concentrations slowly decrease over the
remainder of the 24-hour period of application. At steady state, trough levels
are approximately 60-80% of peak levels. Fluctuation (between Cmax and Cmin)
is lower for Exelon Patch than for the oral formulation. Exelon Patch 9.5
mg/24 hours exhibited exposure approximately the same as that provided by an
oral dose of 6 mg twice daily (i.e., 12 mg/day).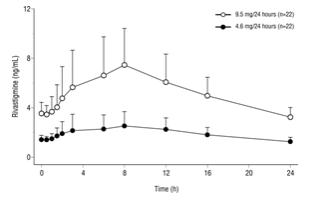
Figure 2: Rivastigmine Plasma Concentrations Following Dermal 24-Hour Patch Application
Inter-subject variability in exposure was lower (43-49%) for the Exelon Patch formulation as compared with the oral formulations (73-103%).
A relationship between drug exposure at steady state (rivastigmine and metabolite NAP226-90) and bodyweight was observed in Alzheimer’s dementia patients. Compared to a patient with a body weight of 65 kg, the rivastigmine steady-state concentrations in a patient with a body weight of 35 kg are approximately doubled, while for a patient with a body weight of 100 kg the concentrations are approximately halved. The effect of body weight on drug exposure suggests special attention to patients with very low body weight during up-titration [see Dosage and Administration (2)].
Over a 24-hour dermal application, approximately 50% of the drug load is released from the system.
Exposure (AUC∞) to rivastigmine (and metabolite NAP266-90) was highest when the patch was applied to the upper back, chest, or upper arm. Two other sites (abdomen and thigh) could be used if none of the three other sites is available, but the practitioner should keep in mind that the rivastigmine plasma exposure associated with these sites was approximately 20-30% lower.
There was no relevant accumulation of rivastigmine or the metabolite NAP226-90 in plasma in patients with Alzheimer’s disease upon multiple dosing.
12.3.2
Distribution
Rivastigmine is weakly bound to plasma proteins (approximately 40%) over the therapeutic range. It readily crosses the blood-brain barrier, reaching CSF peak concentrations in 1.4-2.6 hours. It has an apparent volume of distribution in the range of 1.8-2.7 L/kg.
12.3.3
Metabolism
Rivastigmine is extensively metabolized primarily via cholinesterase-mediated hydrolysis to the decarbamylated metabolite NAP226-90. In vitro, this metabolite shows minimal inhibition of acetylcholinesterase (less than 10%). Based on evidence from in-vitro and animal studies, the major cytochrome P450 isoenzymes are minimally involved in rivastigmine metabolism.
The metabolite-to-parent AUC∞ ratio was about 0.7 after Exelon Patch application versus 3.5 after oral administration, indicating that much less metabolism occurred after dermal treatment. Less NAP226-90 is formed following patch application, presumably because of the lack of presystemic (hepatic first pass) metabolism. Based on in-vitro studies, no unique metabolic routes were detected in human skin.
12.3.4
Elimination
Renal excretion of the metabolites is the major route of elimination.
Unchanged rivastigmine is found in trace amounts in the urine. Following
administration of 14C-rivastigmine, renal elimination was rapid and
essentially complete (greater than 90%) within 24 hours. Less than 1% of the
administered dose is excreted in the feces. The apparent elimination half-life
in plasma is approximately 3 hours after patch removal. Renal clearance was
approximately
2.1-2.8 L/hr.
