First Aid Direct Allergy Relief
First Aid Direct Allergy Relief
6b43e5a5-30a8-46ca-a44d-1e1abf567b51
HUMAN OTC DRUG LABEL
Sep 23, 2025
Cintas Corporation
DUNS: 056481716
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Loratadine Tablets
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
Drug Labeling Information
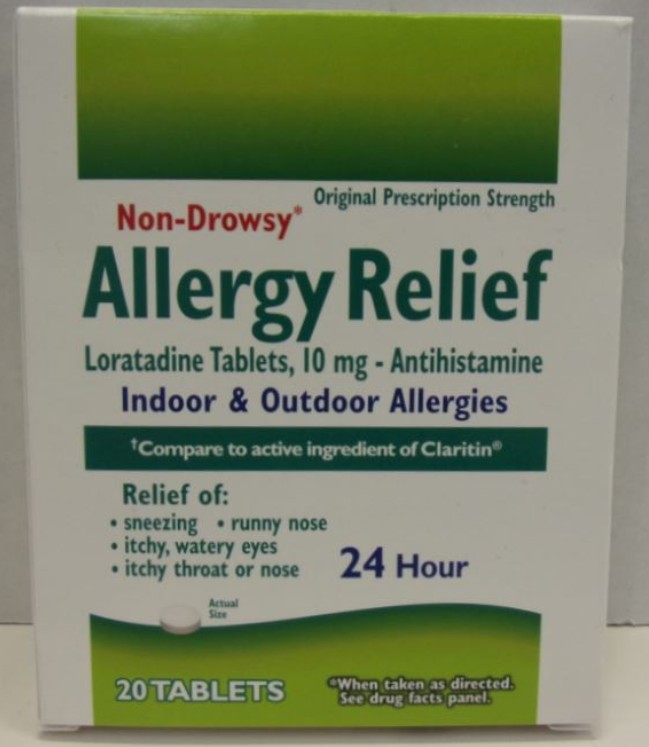
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Principal Display Panel
Original Prescription Strength
Non-Drowsy*
Allergy Relief
Loratadine Tablets, 10 mg - Antihistamine
Indoor & Outdoor Allergies
†Compare to active ingredient of Claritin®
Relief of:
- sneezing
- runny nose
- itchy, watery eyes
- itchy throat or nose
24 Hour
Actual Size
20 TABLETS
*When taken as directed. See drug facts panel
INDICATIONS & USAGE SECTION
Uses
temporatily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- itchy, watery eyes
- sneezing
- iching of the nose or throat
OTC - ACTIVE INGREDIENT SECTION
Active Ingredient (in each tablet)
Loratadine 10 mg
OTC - PURPOSE SECTION
Purpose
Antihistamine
WARNINGS SECTION
Warnings
Do not use
if you have ever had an allergic reaction to this product or any of its ingredients
Ask a doctor before use if you
have liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
do not take more than directed. Taking more than directed may cause drowsiness.
Stop use and ask a doctor if
an allergic reaction to this product occurs.
Seek medical help right away.
If pregnant or breast-feeding,
ask a health professional before use
Keep out of reach of children.
In case of accidental overdose, contact a doctor or Poison Control Center immediately.
DOSAGE & ADMINISTRATION SECTION
Directions
|
** adults and children 6 years and over****** |
1 tablet daily; not more than 1 tablet in 24 hours |
|
** children under 6 years of age** |
ask a doctor |
|
** consumers with liver or kidney disease** |
ask a doctor |
STORAGE AND HANDLING SECTION
Other information
- store between 20°C to 25°C (68° to 77°F)
- protect from excessive moisture
- do not use if blister unit is torn or open
INACTIVE INGREDIENT SECTION
Inactive ingredients
lactose monohydrate, magensium stearate, pregelatinized starch, sodium startch glycolate
OTC - QUESTIONS SECTION
Questions?
1-800-327-2704 Monday - Friday 8:00am - 5:00pm (CDT)
