Paricalcitol
These highlights do not include all the information needed to use PARICALCITOL CAPSULES safely and effectively. See full prescribing information for . s, for oral use
aa1a80e9-8778-a03e-5d53-d4b6a6363169
HUMAN PRESCRIPTION DRUG LABEL
Apr 27, 2017
Dr. Reddy's Laboratories Limited
DUNS: 650562841
Products 3
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Paricalcitol
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
Paricalcitol
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Paricalcitol
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
Drug Labeling Information
RECENT MAJOR CHANGES SECTION
RECENT MAJOR CHANGES
Dosage and Administration, Pediatric Patients 10/2016****
Dosage and Administration, Pediatric Patients 10/2016****
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Chronic Kidney Disease Stages 3 and 4
Adults****
The safety and efficacy of paricalcitol capsules were evaluated in three, 24-week, double blind, placebo-controlled, randomized, multicenter, Phase 3 clinical studies in CKD Stages 3 and 4 patients. Two studies used an identical three times a week dosing design, and one study used a daily dosing design. A total of 107 patients received paricalcitol capsules and 113 patients received placebo. The mean age of the patients was 63 years, 68% were male, 71% were Caucasian, and 26% were African-American. The average baseline iPTH was 274 pg/mL (range: 145 to 856 pg/mL). The average duration of CKD prior to study entry was 5.7 years. At study entry 22% were receiving calcium based phosphate binders and/or calcium supplements. Baseline 25-hydroxyvitamin D levels were not measured.
The initial dose of paricalcitol capsules was based on baseline iPTH. If iPTH was ≤ 500 pg/mL, paricalcitol capsules were administered 1 mcg daily or 2 mcg three times a week, not more than every other day. If iPTH was > 500 pg/mL, paricalcitol capsules were administered 2 mcg daily or 4 mcg three times a week, not more than every other day. The dose was increased by 1 mcg daily or 2 mcg three times a week every 2 to 4 weeks until iPTH levels were reduced by at least 30% from baseline. The overall average weekly dose of paricalcitol capsules was 9.6 mcg/week in the daily regimen and 9.5 mcg/week in the three times a week regimen.
In the clinical studies, doses were titrated for any of the following reasons: if iPTH fell to < 60 pg/mL, or decreased > 60% from baseline, the dose was reduced or temporarily withheld; if iPTH decreased < 30% from baseline and serum calcium was ≤ 10.3 mg/dL and serum phosphorus was ≤ 5.5 mg/dL, the dose was increased; and if iPTH decreased between 30 to 60% from baseline and serum calcium and phosphorus were ≤ 10.3 mg/dL and ≤ 5.5 mg/dL, respectively, the dose was maintained. Additionally, if serum calcium was between 10.4 to 11.0 mg/dL, the dose was reduced irrespective of iPTH, and the dose was withheld if serum calcium was > 11 mg/dL. If serum phosphorus was > 5.5 mg/dL, dietary counseling was provided, and phosphate binders could have been initiated or increased. If the elevation persisted, the paricalcitol Capsules dose was decreased. Seventy-seven percent (77%) of the paricalcitol capsules treated patients and 82% of the placebo treated patients completed the 24-week treatment. The primary efficacy endpoint of at least two consecutive ≥ 30% reductions from baseline iPTH was achieved by 91% of paricalcitol capsules treated patients and 13% of the placebo treated patients (p < 0.001). The proportion of paricalcitol capsules treated patients achieving two consecutive ≥ 30% reductions was similar between the daily and the three times a week regimens (daily: 30/33, 91%; three times a week: 62/68, 91%).
The incidence of hypercalcemia (defined as two consecutive serum calcium values > 10.5 mg/dL), and hyperphosphatemia in paricalcitol capsules treated patients was similar to placebo. There were no treatment related adverse events associated with hypercalcemia or hyperphosphatemia in the paricalcitol capsules group. No increases in urinary calcium or phosphorous were detected in paricalcitol capsules treated patients compared to placebo.
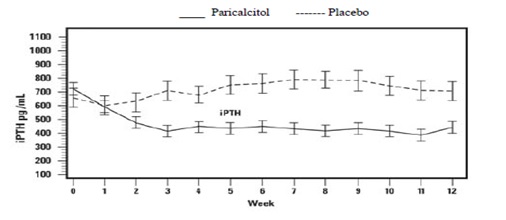
The pattern of change in the mean values for serum iPTH during the studies is shown in Figure 1.
Figure 1. Mean Values for Serum iPTH Over Time in the Three Double-Blind, Placebo-Controlled, Phase 3, CKD Stages 3 and 4 Studies Combined
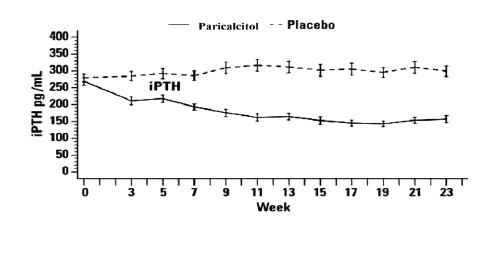
The mean changes from baseline to final treatment visit in serum iPTH, calcium, phosphorus, and bone-specific alkaline phosphatase are shown in Table 9.
Table 9. Mean Changes from Baseline to Final Treatment Visit in Serum iPTH, Bone Specific Alkaline Phosphatase, Calcium, Phosphorus, and Calcium x Phosphorus Product in Three Combined Double-Blind, Placebo-Controlled, Phase 3, CKD Stages 3 and 4 Studies
|
Paricalcitol Capsules |
Placebo**** | |
|
iPTH (pg/mL) |
n = 104 |
n = 110 |
|
Mean Baseline Value |
266 |
279 |
|
Mean Final Treatment Value |
162 |
315 |
|
Mean Change from Baseline (SE) |
-104 (9.2) |
+35 (9) |
|
Bone Specific Alkaline Phosphatase (mcg/L) |
n = 101 |
n = 107 |
|
Mean Baseline |
17.1 |
18.8 |
|
Mean Final Treatment Value |
9.2 |
17.4 |
|
Mean Change from Baseline (SE) |
-7.9 (0.76) |
-1.4 (0.74) |
|
Calcium (mg/dL) |
n = 104 |
n = 110 |
|
Mean Baseline |
9.3 |
9.4 |
|
Mean Final Treatment Value |
9.5 |
9.3 |
|
Mean Change from Baseline (SE) |
+0.2 (0.04) |
-0.1 (0.04) |
|
Phosphorus (mg/dL) |
n = 104 |
n = 110 |
|
Mean Baseline |
4 |
4 |
|
Mean Final Treatment Value |
4.3 |
4.3 |
|
Mean Change from Baseline (SE) |
+0.3 (0.08) |
+0.3 (0.08) |
Pediatric use information for patients 10 to 16 years of age is approved for AbbVie Inc.’s Zemplar (paricalcitol) capsules. However, due to AbbVie Inc.’s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
14.2 Chronic Kidney Disease Stage 5
Adults
The safety and efficacy of paricalcitol capsules were evaluated in a Phase 3, 12-week, double blind, placebo-controlled, randomized, multicenter study in patients with CKD Stage 5 on HD or PD. The study used a three times a week dosing design. A total of 61 patients received paricalcitol capsules and 27 patients received placebo. The mean age of the patients was 57 years, 67% were male, 50% were Caucasian, 45% were African-American, and 53% were diabetic. The average baseline serum iPTH was 701 pg/mL (range: 216-1933 pg/mL). The average time since first dialysis across all subjects was 3.3 years.
The initial dose of paricalcitol capsules was based on baseline iPTH/60. Subsequent dose adjustments were based on iPTH/60 as well as primary chemistry results that were measured once a week. Starting at Treatment Week 2, study drug was maintained, increased or decreased weekly based on the results of the previous week’s calculation of iPTH/60. paricalcitol capsules were administered three times a week, not more than every other day.
The proportion of patients achieving at least two consecutive weekly ≥ 30% reductions from baseline serum iPTH was 88% of paricalcitol capsules treated patients and 13% of the placebo treated patients. The proportion of patients achieving at least two consecutive weekly ≥ 30% reductions from baseline iPTH was similar for HD and PD patients.
The incidence of hypercalcemia (defined as two consecutive serum calcium values > 10.5 mg/dL) in patients treated with paricalcitol capsules was 6.6% as compared to 0% for patients given placebo. In PD patients the incidence of hypercalcemia in patients treated with paricalcitol capsules was 21% as compared to 0% for patients given placebo. The patterns of change in the mean values for serum iPTH are shown in Figure 2. The rate of hypercalcemia with paricalcitol capsules may be reduced with a lower dosing regimen based on the iPTH/80 formula as shown by computer simulations. The hypercalcemia rate can be further predicted to decrease, if the treatment is initiated in only those with baseline serum calcium ≤ 9.5 mg/dL [see Clinical Pharmacology (12.2) and Dosage and Administration (2.2)].
**Figure 2. Mean Values for Serum iPTH Over Time in a Phase 3, Double-Blind, Placebo-**Controlled CKD Stage 5 Study