Lovastatin
LOVASTATIN TABLETS USP 10 mg, 20 mg and 40 mg Rx only
7b86160d-74a9-4774-b5b3-5641e686991f
HUMAN PRESCRIPTION DRUG LABEL
Jul 21, 2023
BluePoint Laboratories
DUNS: 985523874
Products 3
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
lovastatin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
lovastatin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
lovastatin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
Drug Labeling Information
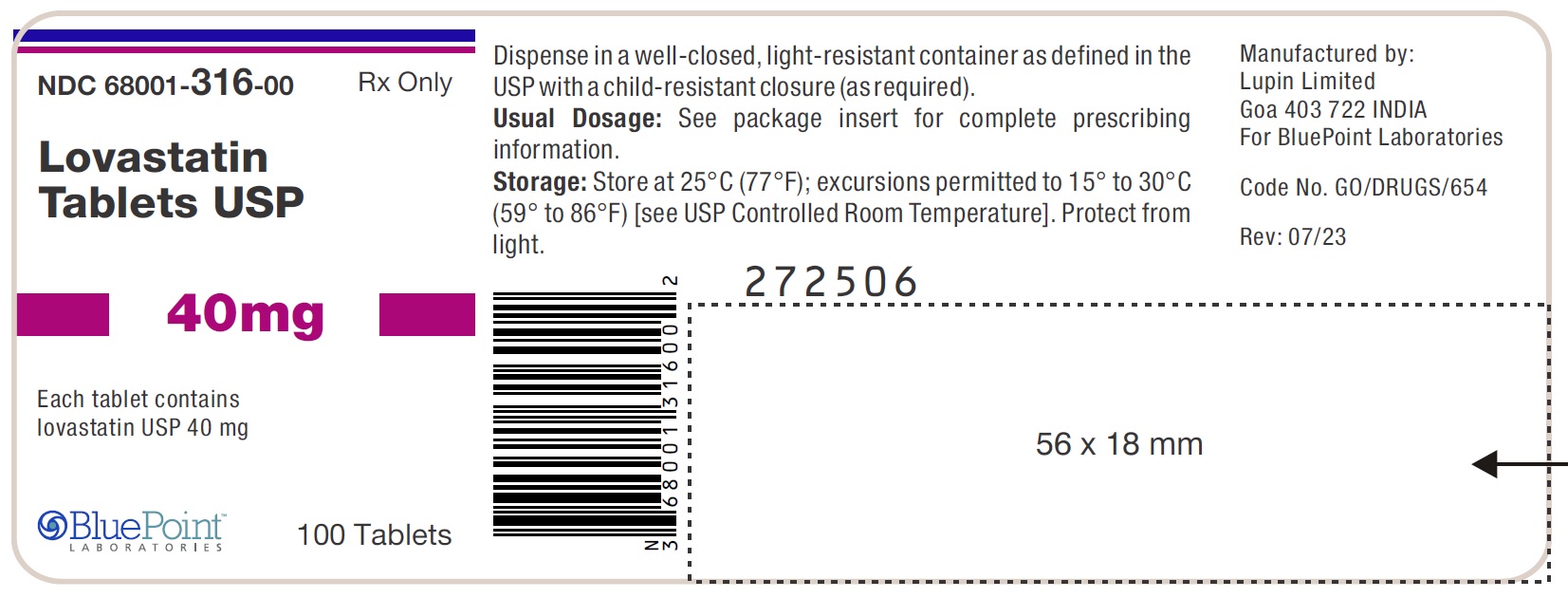
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Package/Label Display Panel
NDC 68001-316-00
LOVASTATIN TABLETS USP
40 mg
Rx only
Bottle of 100 Tablets
ADVERSE REACTIONS SECTION
ADVERSE REACTIONS
Phase III Clinical Studies
In Phase III controlled clinical studies involving 613 patients treated with lovastatin, the adverse experience profile was similar to that shown below for the 8,245-patient EXCEL study (see Expanded Clinical Evaluation of Lovastatin [EXCEL] Study).
Persistent increases of serum transaminases have been noted (see WARNINGS, Liver Dysfunction). About 11% of patients had elevations of CK levels of at least twice the normal value on one or more occasions. The corresponding values for the control agent cholestyramine was 9 percent. This was attributable to the noncardiac fraction of CK. Large increases in CK have sometimes been reported (see WARNINGS, Myopathy/Rhabdomyolysis).
Expanded Clinical Evaluation of Lovastatin (EXCEL) Study
Lovastatin was compared to placebo in 8,245 patients with hypercholesterolemia (total-C 240 to 300 mg**/dL [6.2 to 7.8 mmol/**L]) in the randomized, double-blind, parallel, 48-week EXCEL study. Clinical adverse experiences reported as possibly, probably or definitely drug-related in ≥1% in any treatment group are shown in the table below. For no event was the incidence on drug and placebo statistically different.
|
Placebo (N =1663) |
Lovastatin |
Lovastatin |
Lovastatin |
Lovastatin | |
|
Body As a Whole | |||||
|
Asthenia |
1.4 |
1.7 |
1.4 |
1.5 |
1.2 |
|
Gastrointestinal | |||||
|
Abdominal pain |
1.6 |
2.0 |
2.0 |
2.2 |
2.5 |
|
Constipation |
1.9 |
2.0 |
3.2 |
3.2 |
3.5 |
|
Diarrhea |
2.3 |
2.6 |
2.4 |
2.2 |
2.6 |
|
Dyspepsia |
1.9 |
1.3 |
1.3 |
1.0 |
1.6 |
|
Flatulence |
4.2 |
3.7 |
4.3 |
3.9 |
4.5 |
|
Nausea |
2.5 |
1.9 |
2.5 |
2.2 |
2.2 |
|
Musculoskeletal | |||||
|
Muscle cramps |
0.5 |
0.6 |
0.8 |
1.1 |
1.0 |
|
Myalgia |
1.7 |
2.6 |
1.8 |
2.2 |
3.0 |
|
Nervous System/ | |||||
|
Dizziness |
0.7 |
0.7 |
1.2 |
0.5 |
0.5 |
|
Headache |
2.7 |
2.6 |
2.8 |
2.1 |
3.2 |
|
Skin | |||||
|
Rash |
0.7 |
0.8 |
1.0 |
1.2 |
1.3 |
|
Special Senses | |||||
|
Blurred vision |
0.8 |
1.1 |
0.9 |
0.9 |
1.2 |
Other clinical adverse experiences reported as possibly, probably or definitely drug-related in 0.5 to 1.0 percent of patients in any drug-treated group are listed below. In all these cases the incidence on drug and placebo was not statistically different.Body as a Whole: chest pain; Gastrointestinal: acid regurgitation, dry mouth, vomiting; Musculoskeletal: leg pain, shoulder pain, arthralgia;Nervous System/Psychiatric: insomnia, paresthesia;Skin: alopecia, pruritus; Special Senses: eye irritation.
In the EXCEL study (see CLINICAL PHARMACOLOGY, Clinical Studies) , 4.6% of the patients treated up to 48 weeks were discontinued due to clinical or laboratory adverse experiences which were rated by the investigator as possibly, probably or definitely related to therapy with lovastatin. The value for the placebo group was 2.5%.
**Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS) **
In AFCAPS/TexCAPS (see CLINICAL PHARMACOLOGY, Clinical Studies) involving 6,605 participants treated with 20 to 40 mg/day of lovastatin (n=3,304) or placebo (n=3,301), the safety and tolerability profile of the group treated with lovastatin was comparable to that of the group treated with placebo during a median of 5.1 years of follow-up. The adverse experiences reported in AFCAPS/TexCAPS were similar to those reported in EXCEL (see ADVERSE REACTIONS, Expanded Clinical Evaluation of Lovastatin (EXCEL) Study).
Concomitant Therapy
In controlled clinical studies in which lovastatin was administered concomitantly with cholestyramine, no adverse reactions peculiar to this concomitant treatment were observed. The adverse reactions that occurred were limited to those reported previously with lovastatin or cholestyramine. Other lipid-lowering agents were not administered concomitantly with lovastatin during controlled clinical studies. Preliminary data suggests that the addition of gemfibrozil to therapy with lovastatin is not associated with greater reduction in LDL-C than that achieved with lovastatin alone. In uncontrolled clinical studies, most of the patients who have developed myopathy were receiving concomitant therapy with cyclosporine, gemfibrozil or niacin (nicotinic acid). The combined use of lovastatin with cyclosporine or gemfibrozil should be avoided. Caution should be used when prescribing other fibrates or lipid-lowering doses (≥1 g/day) of niacin with lovastatin (see WARNINGS, Myopathy/Rhabdomyolysis).
The following effects have been reported with drugs in this class. Not all the effects listed below have necessarily been associated with lovastatin therapy.
**Skeletal:**muscle cramps, myalgia, myopathy, rhabdomyolysis, arthralgias.
There have been rare reports of immune-mediated necrotizing myopathy associated with statin use (see WARNINGS, Myopathy/Rhabdomyolysis).
**Neurological:**dysfunction of certain cranial nerves (including alteration of taste, impairment of extra-ocular movement, facial paresis), tremor, dizziness, vertigo, paresthesia, peripheral neuropathy, peripheral nerve palsy, psychic disturbances, anxiety, insomnia, depression.
There have been rare postmarketing reports of cognitive impairment (e.g., memory loss, forgetfulness, amnesia, memory impairment, confusion) associated with statin use. These cognitive issues have been reported for all statins. The reports are generally nonserious, and reversible upon statin discontinuation, with variable times to symptom onset (1 day to years) and symptom resolution (median of 3 weeks).
**Hypersensitivity Reactions:**An apparent hypersensitivity syndrome has been reported rarely which has included one or more of the following features: anaphylaxis, angioedema, lupus erythematous-like syndrome, polymyalgia rheumatica, dermatomyositis, vasculitis, purpura, thrombocytopenia, leukopenia, hemolytic anemia, positive ANA, ESR increase, eosinophilia, arthritis, arthralgia, urticaria, asthenia, photosensitivity, fever, chills, flushing, malaise, dyspnea, toxic epidermal necrolysis, erythema multiforme, including Stevens-Johnson syndrome.
**Gastrointestinal:**pancreatitis, hepatitis, including chronic active hepatitis, cholestatic jaundice, fatty change in liver; and rarely, cirrhosis, fulminant hepatic necrosis, and hepatoma; anorexia, vomiting, fatal and non- fatal hepatic failure.
**Skin:alopecia, pruritus. A variety of skin changes (e.g., nodules, discoloration, dryness of skin/**mucous membranes, changes to hair **/**nails) have been reported.
**Reproductive:**gynecomastia, loss of libido, erectile dysfunction.
**Eye:**progression of cataracts (lens opacities), ophthalmoplegia.
**Laboratory Abnormalities:**elevated transaminases, alkaline phosphatase, γ-glutamyl transpeptidase, and bilirubin; thyroid function abnormalities.
Respiratory: interstitial lung disease
Adolescent Patients (ages 10 to 17 years)
In a 48-week controlled study in adolescent boys with heFH (n=132) and a 24-week controlled study in girls who were at least 1 year post-menarche with heFH (n=54), the safety and tolerability profile of the groups treated with lovastatin (10 to 40 mg daily) was generally similar to that of the groups treated with placebo (see CLINICAL PHARMACOLOGY, Clinical Studies in Adolescent Patients and PRECAUTIONS, Pediatric Use).
WARNINGS SECTION
WARNINGS
Myopathy/Rhabdomyolysis
Lovastatin, like other inhibitors of HMG-CoA reductase, occasionally causes myopathy manifested as muscle pain, tenderness or weakness with creatine kinase (CK) above ten times the upper limit of normal (ULN). Myopathy sometimes takes the form of rhabdomyolysis with or without acute renal failure secondary to myoglobinuria, and rare fatalities have occurred. The risk of myopathy is increased by high levels of HMG-CoA reductase inhibitory activity in plasma.
**The risk of myopathy/rhabdomyolysis is dose related.**In a clinical study (EXCEL) in which patients were carefully monitored and some interacting drugs were excluded, there was one case of myopathy among 4933 patients randomized to lovastatin 20 to 40 mg daily for 48 weeks, and 4 among 1649 patients randomized to 80 mg daily.
**All patients starting therapy with lovastatin, or whose dose of lovastatin is being increased, should be advised of the risk of myopathy and told to report promptly any unexplained muscle pain, tenderness or weakness particularly if accompanied by malaise or fever or if muscle signs and symptoms persists after discontinuing lovastatin. Lovastatin therapy should be discontinued immediately if myopathy is diagnosed or suspected.**In most cases, muscle symptoms and CK increases resolved when treatment was promptly discontinued. Periodic CK determinations may be considered in patients starting therapy with lovastatin or whose dose is being increased, but there is no assurance that such monitoring will prevent myopathy.
Many of the patients who have developed rhabdomyolysis on therapy with lovastatin have had complicated medical histories, including renal insufficiency usually as a consequence of long-standing diabetes mellitus. Such patients merit closer monitoring. Lovastatin therapy should be discontinued if markedly elevated CPK levels occur or myopathy is diagnosed or suspected. Lovastatin therapy should also be temporarily withheld in any patient experiencing an acute or serious condition predisposing to the development of renal failure secondary to rhabdomyolysis, e.g., sepsis; hypotension; major surgery; trauma; severe metabolic, endocrine, or electrolyte disorders; or uncontrolled epilepsy.
The risk of myopathy/rhabdomyolysis is increased by concomitant use of lovastatin with the following:
Strong inhibitors of CYP3A4
Lovastatin, like several other inhibitors of HMG-CoA reductase, is a substrate of cytochrome P450 3A4 (CYP3A4). Certain drugs which inhibit this metabolic pathway can raise the plasma levels of lovastatin and may increase the risk of myopathy. These include itraconazole, ketoconazole, posaconazole, voriconazole, the macrolide antibiotics erythromycin and clarithromycin, the ketolide antibiotic telithromycin, HIV protease inhibitors, boceprevir, telaprevir, the antidepressant nefazodone, or cobicistat-containing products. Combination of these drugs with lovastatin is contraindicated. If short-term treatment with strong CYP3A4 inhibitors is unavoidable, therapy with lovastatin should be suspended during the course of treatment (see CONTRAINDICATIONS; PRECAUTIONS, Drug Interactions).
Gemfibrozil
The combined use of lovastatin with gemfibrozil should be avoided.
Other lipid-lowering drugs (other fibrates or ≥1 g/day of niacin)
Caution should be used when prescribing other fibrates or lipid-lowering doses (≥1 g/day) of niacin with lovastatin, as these agents can cause myopathy when given alone.The benefit of further alterations in lipid levels by the combined use of lovastatin with other fibrates or niacin should be carefully weighed against the potential risks of these combinations.
Cyclosporine
The use of lovastatin with cyclosporine should be avoided.
Danazol, diltiazem, dronedarone or verapamil with higher doses of lovastatin
**The dose of lovastatin should not exceed 20 mg daily in patients receiving concomitant medication with danazol, diltiazem, dronedarone, or verapamil. **The benefits of the use of lovastatin in patients receiving danazol, diltiazem, dronedarone, or verapamil should be carefully weighed against the risks of these combinations.
Amiodarone
**The dose of lovastatin should not exceed 40 mg daily in patients receiving concomitant medication with amiodarone.**The combined use of lovastatin at doses higher than 40 mg daily with amiodarone should be avoided unless the clinical benefit is likely to outweigh the increased risk of myopathy. The risk of myopathy/rhabdomyolysis is increased when amiodarone is used concomitantly with higher doses of a closely related member of the HMG-CoA reductase inhibitor class.
Colchicine
Cases of myopathy, including rhabdomyolysis, have been reported with lovastatin coadministered with colchicine, and caution should be exercised when prescribing lovastatin with colchicine (see PRECAUTIONS, Drug Interactions).
Ranolazine
The risk of myopathy, including rhabdomyolysis, may be increased by concomitant administration of ranolazine. Dose adjustment of lovastatin may be considered during coadministration with ranolazine.
Prescribing recommendations for interacting agents are summarized in Table VII (see also CLINICAL PHARMACOLOGY, Pharmacokinetics; PRECAUTIONS, Drug Interactions; DOSAGE AND ADMINISTRATION).
|
Table VII Drug Interactions Associated with Increased Risk of Myopathy/ Rhabdomyolysis | |
|
Interacting Agents |
Prescribing Recommendations |
|
Strong CYP3A4 inhibitors, e.g.: Ketoconazole Itraconazole Posaconazole Voriconazole Erythromycin Clarithromycin Telithromycin HIV protease inhibitors Boceprevir Telaprevir Nefazodone Cobicistat-containing products |
Contraindicated with lovastatin |
|
Gemfibrozil Cyclosporine |
Avoid with lovastatin |
|
Danazol Diltiazem Dronedarone Verapamil |
Do not exceed 20 mg lovastatin daily |
|
Amiodarone |
Do not exceed 40 mg lovastatin daily |
|
Grapefruit juice |
Avoid grapefruit juice |
Immune-Mediated Necrotizing Myopathy
There have been rare reports of immune-mediated necrotizing myopathy (IMNM), an autoimmune myopathy, associated with statin use. IMNM is characterized by: proximal muscle weakness and elevated serum creatine kinase, which persist despite discontinuation of statin treatment; positive anti-HMG CoA reductase antibody; muscle biopsy showing necrotizing myopathy; and improvement with immunosuppressive agents. Additional neuromuscular and serologic testing may be necessary. Treatment with immunosuppressive agents may be required. Consider risk of IMNM carefully prior to initiation of a different statin. If therapy is initiated with a different statin, monitor for signs and symptoms of IMNM.
Liver Dysfunction
Persistent increases (to more than 3 times the upper limit of normal) in serum transaminases occurred in 1.9% of adult patients who received lovastatin for at least one year in early clinical trials(see ADVERSE REACTIONS). When the drug was interrupted or discontinued in these patients, the transaminase levels usually fell slowly to pretreatment levels. The increases usually appeared 3 to 12 months after the start of therapy with lovastatin, and were not associated with jaundice or other clinical signs or symptoms. There was no evidence of hypersensitivity. In the EXCEL study (see CLINICAL PHARMACOLOGY, Clinical Studies) , the incidence of persistent increases in serum transaminases over 48 weeks was 0.1% for placebo, 0.1% at 20 mg/day, 0.9% at 40 mg/day, and 1.5% at 80 mg/day in patients on lovastatin. However, in post-marketing experience with lovastatin, symptomatic liver disease has been reported rarely at all dosages (see ADVERSE REACTIONS).
In AFCAPS/TexCAPS, the number of participants with consecutive elevations of either alanine aminotransferase (ALT) or aspartate aminotransferase (AST) (> 3 times the upper limit of normal), over a median of 5.1 years of follow-up, was not significantly different between the lovastatin and placebo groups (18 [0.6%] vs. 11 [0.3%]). The starting dose of lovastatin was 20 mg/day; 50% of the lovastatin treated participants were titrated to 40 mg/day at Week 18. Of the 18 participants on lovastatin with consecutive elevations of either ALT or AST, 11 (0.7%) elevations occurred in participants taking 20 mg/day, while 7 (0.4%) elevations occurred in participants titrated to 40 mg/day. Elevated transaminases resulted in discontinuation of 6 (0.2%) participants from therapy in the lovastatin group (n=3,304) and 4 (0.1%) in the placebo group (n=3,301).
It is recommended that liver enzyme tests be obtained prior to initiating therapy with lovastatin and repeated as clinically indicated.
There have been rare postmarketing reports of fatal and non-fatal hepatic failure in patients taking statins, including lovastatin. If serious liver injury with clinical symptoms and/or hyperbilirubinemia or jaundice occurs during treatment with lovastatin, promptly interrupt therapy. If an alternate etiology is not found do not restart lovastatin.
The drug should be used with caution in patients who consume substantial quantities of alcohol and/or have a past history of liver disease. Active liver disease or unexplained transaminase elevations are contraindications to the use of lovastatin.
Moderate (less than three times the upper limit of normal) elevations of serum transaminases have been reported following therapy with lovastatin (see ADVERSE REACTIONS). These changes appeared soon after initiation of therapy with lovastatin, were often transient, were not accompanied by any symptoms and interruption of treatment was not required.
HOW SUPPLIED SECTION
HOW SUPPLIED
Lovastatin Tablets USP, 10 mg are light green colored, oval shaped, uncoated tablets, debossed with 'LU' on one side and 'G01' on the other side. They are supplied as follows:
NDC 68001-314-00 Bottles of 100
NDC 68001-314-08 Bottles of 1000
Lovastatin Tablets USP, 20 mg are light green colored, circular, beveled edged, uncoated tablets, debossed with 'LU' on one side and 'G02' on the other side. They are supplied as follows:
NDC 68001-315-00 Bottles of 100
NDC 68001-315-08 Bottles of 1000
Lovastatin Tablets USP, 40 mg are light green colored, circular, beveled edged uncoated tablets, debossed with 'LU' on one side and 'G03' on the other side. They are supplied as follows:
NDC 68001-316-00 Bottles of 100
NDC 68001-316-08 Bottles of 1000
Storage
Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Lovastatin tablets must be protected from light and stored in a well-closed, light-resistant container.
1 Kantola, T, et al., Clin Pharmacol Ther 1998; 63(4): 397-402
3 Manson, J.M., Freyssinges,C.Ducrocq, M.B., Stephenson, W.P., Postmarketing Surveillance of Lovastatin and Simvastatin Exposure During Pregnancy. Reproductive Toxicology. 10(6):439-446, 1996.
4 National Cholesterol Education Program (NCEP): Highlights of the Report of the Expert Panel on Blood Cholesterol Levels in Children and Adolescents. Pediatrics. 89(3):495-501. 1992.
Manufactured by:
Lupin Limited
Goa 403 722 INDIA
For BluePoint Laboratories
Revised: 01/2021 ID#: 266229
