Cold Flu Relief Elderberry
Cold + Flu Relief Elderberry Syrup
27ec0362-b762-442e-ad82-1f17fe15858e
HUMAN OTC DRUG LABEL
Sep 9, 2025
Schwabe North America
DUNS: 831153908
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Cold Flu Relief Elderberry
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (18)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
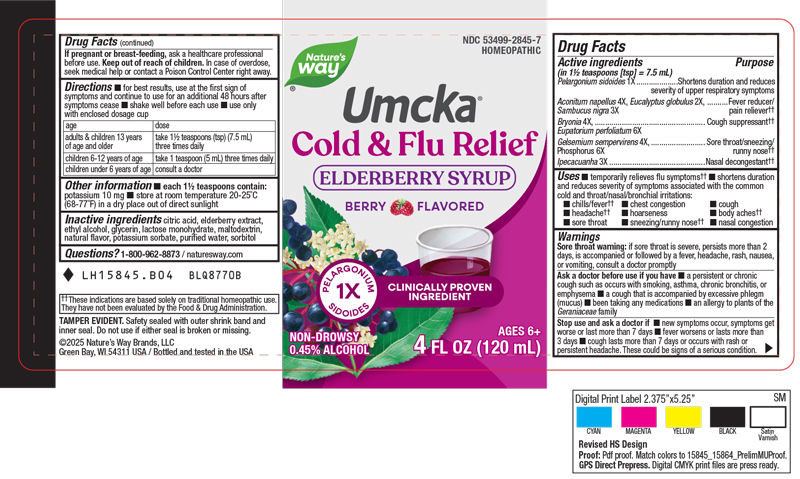

INDICATIONS & USAGE SECTION
Indications & Usage
Temporarily relieves flu symptoms.
Shortens duration and reduces severity of symptoms associated with the common cold and throat/nasal/bronchial irritations: chills/fever, chest congestion, cough, headache, hoarseness, body aches, sore throat, sneezing, stuffy/runny nose, nasal congestion.
OTC - ACTIVE INGREDIENT SECTION
Active Ingredients
Pelargonium sidoides 1x
Aconitum napellus 4x
Eucalyptus globulus 2x
Sambucus nigra 3x
Bryonia 4x
Eupatorium perfoliatum 6x
Gelsemium sempervirens 4x
Phosphorus 6x
Ipecacuanha 3x
INACTIVE INGREDIENT SECTION
Inactive Ingredients
Citric Acid
Elderberry Extract
Ethyl Alcohol
Glycerin
Lactose Monohydrate
Maltodextrin
Natural Flavor
Potassium Sorbate
Purified Water
Sorbitol
DOSAGE & ADMINISTRATION SECTION
Dosage & Administration
Directions:
For best results, use at the first sign of symptoms and continue to use for an additional 48 hours after symptoms cease.
Shake well before each use.
Use only with enclosed dosage cup.
Adults & children 13 years of age and older: take 1½ teaspoons (tsp) (7.5 mL) three times daily.
Children 6 to 12 years of age: take 1 teaspoon (tsp) (5mL) three times daily.
Children under 6 years of age: consult a doctor.
OTC - PURPOSE SECTION
Purpose
Temporarily relieves flu symptoms.
Shortens duration and reduces severity of symptoms associated with the common cold and throat/nasal/bronchial irritations: chills/fever, chest congestion, cough, headache, hoarseness, body aches, sore throat, sneezing, stuffy/runny nose, nasal congestion.
WARNINGS SECTION
Warnings
Sore throat warning: if sore throat is severe or persists more than 2 days, is accompanied or followed by a fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
OTC - ASK DOCTOR SECTION
Ask Doctor
Ask a doctor before use if you have: a persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema, a cough that is accompanied by excessive phlegm (mucus), been taking any medications, an allergy to plants of the Geraniaceae family.
OTC - STOP USE SECTION
Stop Use
Stop use and ask a doctor if new symptoms occur, symptoms get worse or last more than 7 days, fever worsens or lasts more than 3 days, cough lasts more then 7 days or occurs with rash or persistent headache.
These could be signs of a serious condition.
OTC - PREGNANCY OR BREAST FEEDING SECTION
Pregnancy or Breastfeeding
If pregnant or breast-feeding, ask a healthcare professional before use.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep out of reach of children
Keep out of reach of children.
OVERDOSAGE SECTION
Overdose
In case of overdose, seek medical help or contact a Poison Control Center right away.
