Barhemsys
These highlights do not include all the information needed to use BARHEMSYS safely and effectively. See full prescribing information for BARHEMSYS. BARHEMSYS (amisulpride) injection, for intravenous use Initial U.S. Approval: 2020
ab23bc6e-b6a8-165f-e053-2a95a90ab144
HUMAN PRESCRIPTION DRUG LABEL
Sep 26, 2022
Acacia Pharma Ltd
DUNS: 779660930
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Amisulpride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
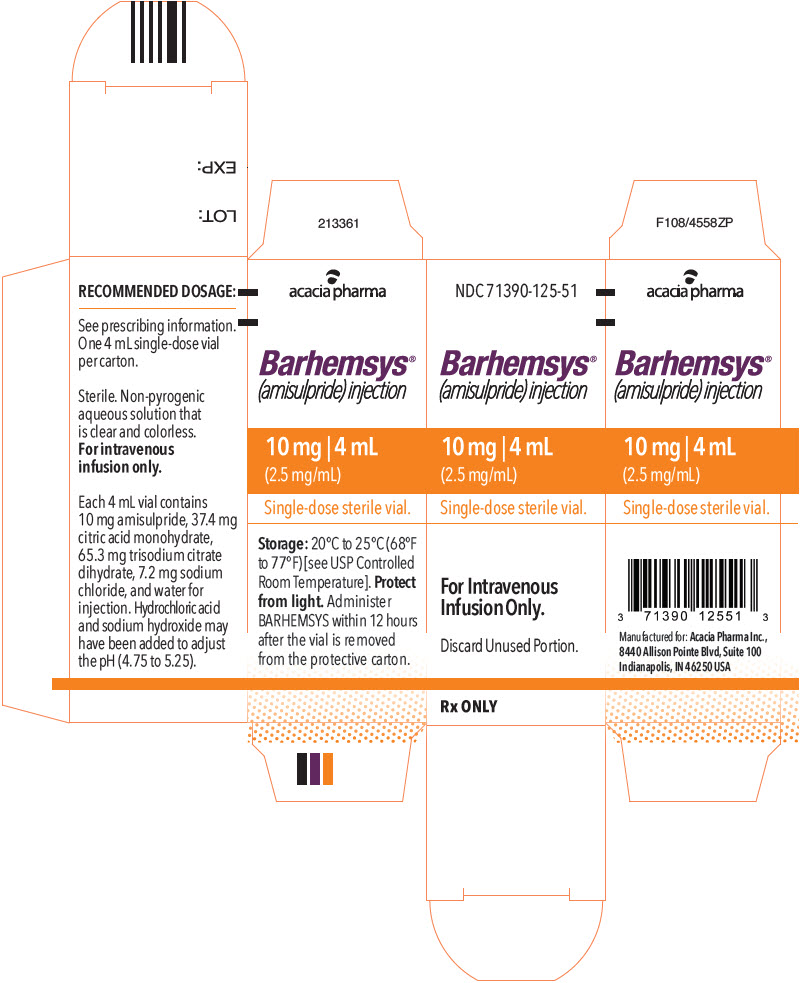
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 4 mL Vial Carton
NDC 71390-125-51
Barhemsys®
(amisulpride) injection
10 mg | 4 mL
(2.5 mg/mL)
Single-dose sterile vial.
For Intravenous
Infusion Only.
Discard Unused Portion.
Rx ONLY
DESCRIPTION SECTION
11 DESCRIPTION
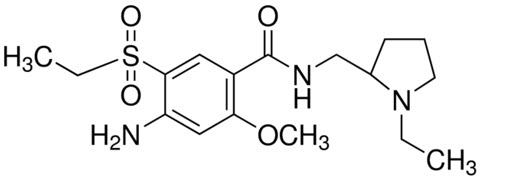
The active ingredient of BARHEMSYS is amisulpride, a dopamine-2 (D2) receptor antagonist. Its chemical name is 4-Amino-N-[(1-ethyl-2-pyrrolidinyl)methyl]-5-(ethylsulfonyl)-o-anisamide. It has the following chemical structure:
|
|
(racemic) |
The empirical formula is C17H27N3O4S representing a molecular weight of 369.48.
Amisulpride is a white or almost white crystalline powder. It is practically insoluble in water, sparingly soluble in ethanol and freely soluble in methylene chloride, and has a melting point of around 126°C. The compound is racemic and shows no optical rotation and is not hygroscopic. No other polymorphs of amisulpride have been reported.
BARHEMSYS (amisulpride) injection is a clear, colorless, nonpyrogenic, sterile solution formulation of amisulpride 5 mg/2 mL (2.5 mg/mL) or 10 mg/4 mL (2.5 mg/mL) for intravenous infusion presented in a single-dose vial. It has a pH of approximately 5.0 and the osmolality of the product is between 250 and 330 mOsmol/kg.
Each 2 mL vial of BARHEMSYS contains 5 mg of amisulpride; 18.7 mg of citric acid monohydrate USP; 3.6 mg of sodium chloride USP; 32.64 mg of trisodium citrate dihydrate; hydrochloric acid NF and sodium hydroxide NF as needed to adjust the pH (4.75 to 5.25); and Water for Injection USP to make up to volume.
Each 4 mL vial of BARHEMSYS contains 10 mg of amisulpride; 37.4 mg of citric acid monohydrate USP; 7.2 mg of sodium chloride USP; 65.3 mg of trisodium citrate dihydrate; hydrochloric acid NF and sodium hydroxide NF as needed to adjust the pH (4.75 to 5.25); and Water for Injection USP to make up to volume.