Warfarin Sodium
These highlights do not include all the information needed to use WARFARIN SODIUM TABLETS safely and effectively. See full prescribing information for WARFARIN SODIUM TABLETS. WARFARIN SODIUM tablets, for oral use Initial U.S. Approval: 1954
8ad881e0-ca41-42ad-9d7d-eb85b3a30af0
HUMAN PRESCRIPTION DRUG LABEL
Dec 21, 2023
Sun Pharmaceutical Industries, Inc.
DUNS: 146974886
Taro Pharmaceuticals U.S.A., Inc.
DUNS: 145186370
Products 9
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Warfarin Sodium
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
Warfarin Sodium
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
Warfarin Sodium
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Warfarin Sodium
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Warfarin Sodium
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
Warfarin Sodium
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (4)
Warfarin Sodium
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
Warfarin Sodium
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Warfarin Sodium
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 10 mg Tablet Bottle Label
NDC 51672-4035-1
** 100 Tablets**
Warfarin Sodium
** Tablets, USP**Crystalline
10 mg
Dispense with
** Medication Guide**
PROTECT FROM LIGHT.
HIGHLY POTENT ANTICOAGULANT.
**WARNING:**Serious bleeding results from overdosage.
Do not use or dispense before reading directions and
warnings in accompanying product information.
Rx only

DESCRIPTION SECTION
11 DESCRIPTION
Warfarin sodium tablets contain warfarin sodium, an anticoagulant that acts by inhibiting vitamin K-dependent coagulation factors. The chemical name of warfarin sodium is 3-(α-acetonylbenzyl)-4-hydroxycoumarin sodium salt, which is a racemic mixture of the R- and S-enantiomers. Crystalline warfarin sodium is an isopropanol clathrate. Its empirical formula is C 19H 15NaO 4, and its structural formula is represented by the following:
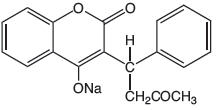
Crystalline warfarin sodium occurs as a white, odorless, crystalline powder that is discolored by light. It is very soluble in water, freely soluble in alcohol, and very slightly soluble in chloroform and ether.
Warfarin sodium tablets, USP for oral use also contain:
All strengths: Anhydrous lactose, corn starch, and magnesium stearate
|
1 mg: |
D&C Red No. 6 Barium Lake |
|
2 mg: |
FD&C Blue No. 2 Aluminum Lake, FD&C Red No. 40 Aluminum Lake |
|
2.5 mg: |
D&C Yellow No. 10 Aluminum Lake, FD&C Blue No. 2 Aluminum Lake |
|
3 mg: |
D&C Yellow No. 10 Aluminum Lake, FD&C Blue No. 2 Aluminum Lake, FD&C Red No. 40 Aluminum Lake |
|
4 mg: |
FD&C Blue No. 1 Aluminum Lake |
|
5 mg: |
D&C Red No. 6 Barium Lake, D&C Yellow No. 10 Aluminum Lake |
|
6 mg: |
D&C Yellow No. 10 Aluminum Lake, FD&C Blue No. 2 Aluminum Lake |
|
7.5 mg: |
D&C Yellow No. 10 Aluminum Lake |
|
10 mg: |
Dye Free |
