GLYBURIDE
Glyburide Tablets, USP 1.25, 2.5, and 5 mg
a4e77a68-fc90-4021-e053-2995a90a87df
HUMAN PRESCRIPTION DRUG LABEL
Jan 20, 2023
Denton Pharma, Inc. DBA Northwind Pharmaceuticals
DUNS: 080355546
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
GLYBURIDE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
Drug Labeling Information
DESCRIPTION SECTION
DESCRIPTION
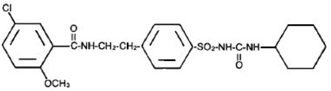
Glyburide tablets, USP contain a smaller particle size glyburide, which is an oral blood-glucose-lowering drug of the sulfonylurea class. Glyburide is a white, crystalline compound, formulated as glyburide tablets, USP of 1.25, 2.5, and 5 mg strengths for oral administration. Inactive ingredients: lactose monohydrate, microcrystalline cellulose, magnesium stearate. In addition, the 2.5 mgcontains FD&C Red No.40 and the5 mg contains FD&C Blue No.1. The chemical name for glyburide is 1-[[p-[2-(5-chloro-o- anisamido)-ethyl]phenyl]-sulfonyl]-3-cyclohexylurea and the molecular weight is 493.99. The structural formula is represented below.