POTASSIUM CITRATE
These highlights do not include all the information needed to use POTASSIUM CITRATE EXTENDED-RELEASE TABLETS safely and effectively. See full prescribing information for POTASSIUM CITRATE EXTENDED-RELEASE TABLETS. POTASSIUM CITRATE Extended-release tablets for oral use Initial U.S. Approval: 1985
44ab2c4f-4a76-470d-98fb-8874c692a967
HUMAN PRESCRIPTION DRUG LABEL
Aug 20, 2025
Bryant Ranch Prepack
DUNS: 171714327
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
POTASSIUM CITRATE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (4)
Drug Labeling Information
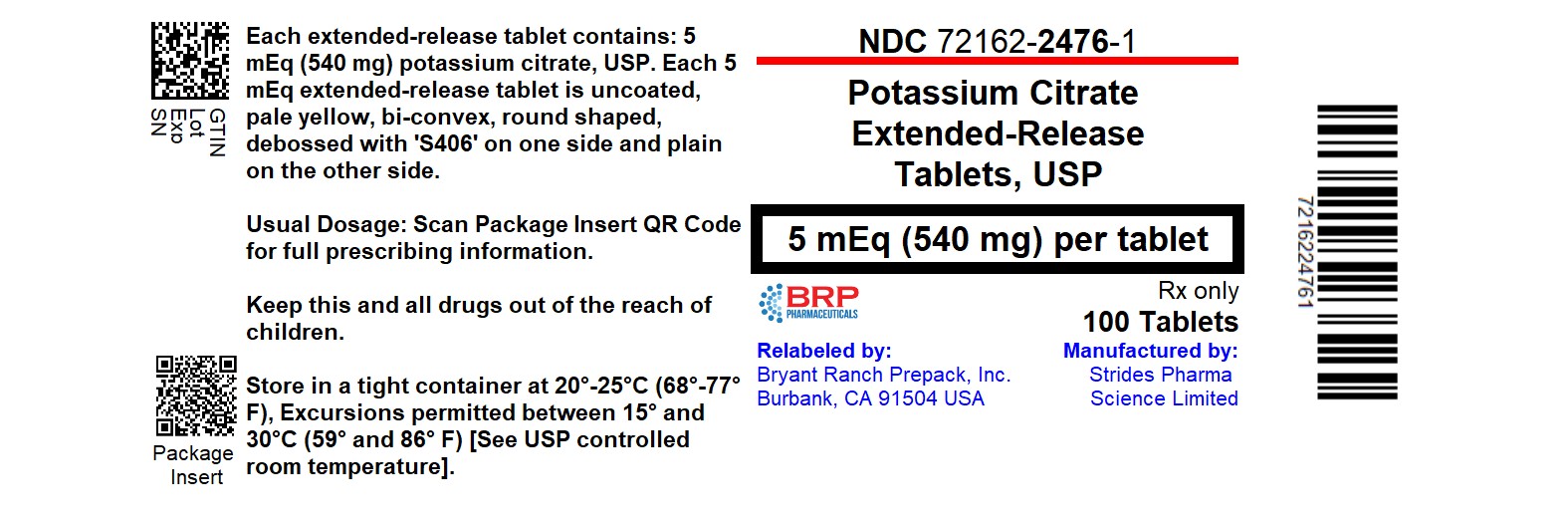
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Potassium Citrate Extended-Release Tablet 5 mEq (540 mg)
DESCRIPTION SECTION
11 DESCRIPTION
Potassium citrate USP is a citrate salt of potassium. Its empirical formula is K 3C 6H 5O 7• H 2O, and it has the following chemical structure:
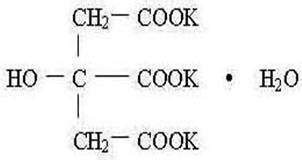
Potassium citrate extended-release tablets USP are pale yellow colored, oral wax-matrix tablets, contain 5 mEq (540 mg) potassium citrate USP, 10 mEq (1080 mg) potassium citrate USP and 15 mEq (1620 mg) potassium citrate USP each. Inactive ingredients include carnauba wax, stearic acid and magnesium stearate.
USP dissolution test is pending.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Potassium citrate extended-release tablets USP 5 mEq are uncoated, pale yellow colored, round shaped, bi-Convex, debossed with 'S406' on one side and plain on the other side, supplied in bottles as:
- NDC 72162-2476-1: 100 Tablets in a BOTTLE
Storage: Store in a tight container at 20°–25°C (68°–77°F), Excursions permitted between 15° and 30°C (59° and 86°F) [See USP controlled room temperature].
Repackaged/Relabeled by:
Bryant Ranch Prepack, Inc.
Burbank, CA 91504
