YUPELRI
These highlights do not include all the information needed to use YUPELRI safely and effectively. See full prescribing information for YUPELRI. YUPELRI (revefenacin) inhalation solution, for oral inhalation Initial U.S. Approval: 2018
6dfebf04-7c90-436a-9b16-750d3c1ee0a6
HUMAN PRESCRIPTION DRUG LABEL
May 3, 2022
Mylan Specialty L.P.
DUNS: 194775557
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
revefenacin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL – 175mcg/3 mL
NDC 49502-806-93
Rx only
YUPELRI**®**
(revefenacin) inhalation solution
175 mcg/3 mL
For Oral Inhalation Only
30 sterile
unit-dose vials
Contents:
Each vial contains 175 mcg of revefenacin in an isotonic, sterile aqueous
solution containing sodium chloride, citric acid and sodium citrate.
Hydrochloric acid or sodium hydroxide may be used to adjust the pH.
Storage:
Store YUPELRI (revefenacin) inhalation solution in the protective foil pouch.
Store at room temperature from 68°F to 77°F (20°C to 25°C); excursions
permitted from 59°F to 86°F (15°C to 30°C) [See USP Controlled Room Temperature]. Protect from direct sunlight and excessive heat. The YUPELRI
unit-dose vial should only be removed from the foil pouch and opened
IMMEDIATELY BEFORE USE. The vial and any residual content should be discarded
after use. Discard any solution that is not clear and colorless. Use only as
directed by your healthcare provider.
Keep out of reach of children. Not a child-resistant package.
Manufactured for:
Mylan Specialty L.P.
Morgantown, WV 26505 USA
Made in USA
Licensed from:
Theravance Biopharma Ireland Limited
TRC:806:30C:R3
© 2021 Viatris Inc.
YUPELRI and the YUPELRI logo are registered trademarks of Mylan Specialty L.P., a Viatris Company.
THERAVANCE® and the Cross/Star logo are registered trademarks of the Theravance Biopharma group of companies.
Patented. See YUPELRI.com/patents

INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
YUPELRI is indicated for the maintenance treatment of patients with chronic obstructive pulmonary disease (COPD).
YUPELRI is an anticholinergic indicated for the maintenance treatment of patients with chronic obstructive pulmonary disease (COPD).
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
YUPELRI is contraindicated in patients with hypersensitivity to revefenacin or any component of this product.
YUPELRI is contraindicated in patients with hypersensitivity to revefenacin or any component of this product. (4)
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Deterioration of Disease and Acute Episodes
YUPELRI should not be initiated in patients during acutely deteriorating or potentially life-threatening episodes of COPD. YUPELRI has not been studied in subjects with acutely deteriorating COPD. The initiation of YUPELRI in this setting is not appropriate.
YUPELRI is intended as a once-daily maintenance treatment for COPD and should not be used for relief of acute symptoms, i.e. as rescue therapy for the treatment of acute episodes of bronchospasm, and extra doses should not be used for that purpose. Acute symptoms should be treated with an inhaled, short-acting beta2-agonist.
COPD may deteriorate acutely over a period of hours or chronically over several days or longer. If YUPELRI no longer controls symptoms of bronchoconstriction, the patient's inhaled, short-acting beta2-agonist becomes less effective, or the patient needs more inhalations of a short-acting beta2-agonist than usual, these may be markers of deterioration of disease. In this setting, a re-evaluation of the patient and the COPD treatment regimen should be undertaken at once. Increasing the daily dose of YUPELRI beyond the recommended dose is not appropriate in this situation.
5.2 Paradoxical Bronchospasm
As with other inhaled medicines, YUPELRI can produce paradoxical bronchospasm that may be life-threatening. If paradoxical bronchospasm occurs following dosing with YUPELRI, it should be treated immediately with an inhaled, short- acting bronchodilator; YUPELRI should be discontinued immediately and alternative therapy should be instituted.
5.3 Worsening of Narrow-Angle Glaucoma
YUPELRI should be used with caution in patients with narrow-angle glaucoma. Prescribers and patients should be alert for signs and symptoms of acute narrow-angle glaucoma (e.g. eye pain or discomfort, blurred vision, visual halos or colored images in association with red eyes from conjunctival congestion and corneal edema). Instruct patients to consult a physician immediately if any of these signs or symptoms develops.
5.4 Worsening of Urinary Retention
YUPELRI should be used with caution in patients with urinary retention. Prescribers and patients should be alert for signs and symptoms of urinary retention (e.g. difficulty passing urine, painful urination), especially in patients with prostatic hyperplasia or bladder-neck obstruction. Instruct patients to consult a healthcare provider immediately if any of these signs or symptoms develops.
5.5 Immediate Hypersensitivity Reactions
Immediate hypersensitivity reactions may occur after administration of YUPELRI. If such a reaction occurs, therapy with YUPELRI should be stopped at once and alternative treatments should be considered.
•
Do not initiate YUPELRI in acutely deteriorating COPD or to treat acute symptoms. (5.1)
•
If paradoxical bronchospasm occurs, discontinue YUPELRI and institute alternative therapy. (5.2)
•
Worsening of narrow-angle glaucoma may occur. Use with caution in patients with narrow-angle glaucoma and instruct patients to contact a healthcare provider immediately if symptoms occur. (5.3)
•
Worsening of urinary retention may occur. Use with caution in patients with prostatic hyperplasia or bladder-neck obstruction and instruct patients to contact a healthcare provider immediately if symptoms occur. (5.4)
•
Immediate hypersensitivity reactions may occur. If such a reaction occurs, therapy with YUPELRI should be stopped at once and alternative treatments should be considered. (5.5)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in labeling:
•
Paradoxical bronchospasm [see Warnings and Precautions (5.2)]
•
Worsening of narrow-angle glaucoma [see Warnings and Precautions (5.3)]
•
Worsening of urinary retention [see Warnings and Precautions (5.4)]
•
Immediate hypersensitivity reactions [see Warnings and Precautions (5.5)]
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The YUPELRI safety database included 2,285 subjects with COPD in two 12-week efficacy studies and one 52-week long-term safety study. A total of 730 subjects received treatment with YUPELRI 175 mcg once daily. The safety data described below are based on the two 12-week trials and the one 52-week trial.
12-Week Trials
YUPELRI was studied in two 12-week replicate placebo-controlled trials in patients with moderate to very severe COPD (Trials 1 and 2). In these trials, 395 patients were treated with YUPELRI at the recommended dose of 175 mcg once daily.
The population had a mean age of 64 years (range from 41 to 88 years), with 50% males, 90% Caucasian, and had COPD with a mean post-bronchodilator forced expiratory volume in one second (FEV1) percent predicted of 55%. Of subjects enrolled in the two 12-week trials, 37% were taking concurrent LABA or ICS/LABA therapy. Patients with unstable cardiac disease, narrow-angle glaucoma, or symptomatic prostatic hypertrophy or bladder outlet obstruction were excluded from these trials.
Table 1 shows the most common adverse reactions that occurred with a frequency of greater than or equal to 2% in the YUPELRI group and higher than placebo in the two 12‑week placebo-controlled trials.
The proportion of subjects who discontinued treatment due to adverse reactions was 13% for the YUPELRI-treated subjects and 19% for placebo-treated subjects.
Table 1: Adverse Reactions with YUPELRI ≥2% Incidence and Higher than Placebo|
Adverse Reaction |
Placebo (N = 418) |
YUPELRI 175 mcg (N = 395) |
|
Respiratory, Thoracic and Mediastinal Disorders | ||
|
Cough |
17 (4%) |
17 (4%) |
|
Infections and Infestations | ||
|
Nasopharyngitis |
9 (2%) |
15 (4%) |
|
Upper respiratory tract infection |
9 (2%) |
11 (3%) |
|
Nervous System Disorders | ||
|
Headache |
11 (3%) |
16 (4%) |
|
Musculoskeletal and Connective Tissue Disorders | ||
|
Back pain |
3 (1%) |
9 (2%) |
Other adverse reactions defined as events with an incidence of ≥1.0%, less than 2.0%, and more common than with placebo included the following: hypertension, dizziness, oropharyngeal pain, and bronchitis.
52-Week Trial
YUPELRI was studied in one 52-week, open-label, active-control (tiotropium 18 mcg once daily) trial in 1,055 patients with COPD. In this trial, 335 patients were treated with YUPELRI 175 mcg once daily and 356 patients with tiotropium. The demographic and baseline characteristics of the long-term safety trial were similar to those of the placebo-controlled 12-week studies described, with the exception that concurrent LABA or LABA/ICS therapy was used in 50% of patients. The adverse reactions reported in the long-term safety trial for YUPELRI were consistent with those observed in the placebo-controlled studies of 12-weeks.
6.2 Postmarketing Experience
The following adverse reaction has been reported during post-approval use of YUPELRI. Because this reaction was reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate the frequency or establish a causal relationship to drug exposure.
Gastrointestinal Disorders: Dry mouth
Most common adverse reactions (incidence greater than or equal to 2% and more common than placebo) include cough, nasopharyngitis, upper respiratory tract infection, headache, and back pain. (6.1)
**To report SUSPECTED ADVERSE REACTIONS, contact Mylan at 1-877-446-3679 (1-877-4-INFO-RX) or FDA at 1-800-FDA-1088 or **www.fda.gov/medwatch.
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Anticholinergics
There is potential for an additive interaction with concomitantly used anticholinergic medicines. Therefore, avoid coadministration of YUPELRI with other anticholinergic-containing drugs as this may lead to an increase in anticholinergic adverse effects [see Warnings and Precautions (5.3, 5.4)].
7.2 Transporter-Related Drug Interactions
OATP1B1 and OATP1B3 inhibitors (e.g. rifampicin, cyclosporine, etc.) could lead to an increase in systemic exposure of the active metabolite. Therefore, coadministration with YUPELRI is not recommended [see Clinical Pharmacology (12.3)].
•
Anticholinergics: May interact additively with concomitantly used anticholinergic medications. Avoid administration of YUPELRI with other anticholinergic-containing drugs. (7.1)
•
Transporter-related drug interactions: Coadministration of YUPELRI with OATP1B1 and OATP1B3 inhibitors (e.g. rifampicin, cyclosporine, etc.) may lead to an increase in exposure of the active metabolite. Therefore, coadministration with YUPELRI is not recommended. (7.2, 12.3)
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
The recommended dosage is 175 mcg YUPELRI (one 175 mcg unit‑dose vial) administered by oral inhalation once daily by nebulizer using a mouthpiece.
Administration Overview
YUPELRI should be administered by the orally inhaled route via a standard jet nebulizer connected to an air compressor (See Instructions for Use). The safety and efficacy of YUPELRI have been established in clinical trials when administered using the PARI LC® Sprint nebulizer with a mouthpiece and the PARI Trek® S compressor. The safety and efficacy of YUPELRI delivered from non‑compressor based nebulizer systems have not been established.
The YUPELRI unit-dose vial should only be removed from the foil pouch and opened IMMEDIATELY BEFORE USE. The vial and any residual content should be discarded after use.
No dosage adjustment is required for geriatric patients, or patients with renal impairment [see Use in Specific Populations (8.5, 8.7) and Clinical Pharmacology (12.3)].
The drug compatibility (physical and chemical), efficacy, and safety of YUPELRI when mixed with other drugs in a nebulizer have not been established.
For oral inhalation use only. Do not swallow YUPELRI.
•
One 175 mcg vial (3 mL) once daily. (2)
•
For use with a standard jet nebulizer with a mouthpiece connected to an air compressor. (2)
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
Inhalation solution: 175 mcg of revefenacin in 3 mL of sterile, clear, colorless, aqueous solution in unit dose vials.
Inhalation solution: 175 mcg of revefenacin per 3 mL solution in unit dose vials. (3)
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate and well-controlled studies with YUPELRI in pregnant women. Women should be advised to contact their physician if they become pregnant while taking YUPELRI. In animal reproduction studies, subcutaneous administration of revefenacin to pregnant rats and rabbits during the period of organogenesis produced no evidence of fetal harm at respective exposures approximately 209 times the exposure at the maximum recommended human dose (MRHD) (on an area under the curve [AUC] basis) (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
In an embryo‑fetal development study in pregnant rats dosed during the period of organogenesis from gestation days 6 to 17, revefenacin was not teratogenic and did not affect fetal survival at exposures up to 209 times the MRHD (based upon summed AUCs for revefenacin and its active metabolite at maternal subcutaneous doses up to 500 mcg/kg/day).
In an embryo‑fetal development study in pregnant rabbits dosed during the period of organogenesis from gestation days 7 to 19, revefenacin was not teratogenic and did not affect fetal survival at exposures up to 694 times the MRHD (based upon summed AUCs for revefenacin and its active metabolite at maternal subcutaneous doses up to 500 mcg/kg/day).
Placental transfer of revefenacin and its active metabolite was observed in pregnant rabbits.
In a pre- and postnatal development (PPND) study in pregnant rats dosed during the periods of organogenesis and lactation from gestation day 6 to lactation day 20, revefenacin had no adverse developmental effects on pups at exposures up to 196 times the MRHD (based upon summed AUCs for revefenacin and its active metabolite at maternal subcutaneous doses up to 500 mcg/kg/day).
8.2 Lactation
Risk Summary
There is no information regarding the presence of revefenacin in human milk, the effects on the breastfed infant, or the effects on milk production. However, revefenacin was present in the milk of lactating rats following dosing during pregnancy and lactation (see Data).
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for YUPELRI and any potential adverse effects on the breastfed infant from YUPELRI or from the underlying maternal condition.
Data
Animal Data
In a PPND study [see Pregnancy (8.1)], revefenacin and its active metabolite were present in milk of lactating rats on lactation day 22. Milk-to-plasma concentration ratios were up to 10 for revefenacin and its active metabolite.
8.4 Pediatric Use
The safety and effectiveness of YUPELRI have not been established in pediatric patients. YUPELRI is not indicated for use in children.
8.5 Geriatric Use
Based on available data, no adjustment of the dosage of YUPELRI in geriatric patients is necessary.
Clinical trials of YUPELRI included 441 subjects aged 65 years and older, and of those, 101 subjects were aged 75 years and older. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
8.6 Hepatic Impairment
The systemic exposure of revefenacin is unchanged while that of its active metabolite is increased in subjects with moderate hepatic impairment. The safety of YUPELRI has not been evaluated in COPD patients with mild-to-severe hepatic impairment. YUPELRI is not recommended in patients with any degree of hepatic impairment. [see Clinical Pharmacology (12.3)].
8.7 Renal Impairment
No dosage adjustment is required in patients with renal impairment. Monitor for systemic antimuscarinic side effects in COPD patients with severe renal impairment. [see Clinical Pharmacology (12.3)].
Hepatic impairment: Avoid use of YUPELRI in patients with hepatic impairment. (8.6, 12.3)
OVERDOSAGE SECTION
10 OVERDOSAGE
An overdosage of YUPELRI may lead to anticholinergic signs and symptoms such as nausea, vomiting, dizziness, lightheadedness, blurred vision, increased intraocular pressure (causing pain, vision disturbances, or reddening of the eye), obstipation or difficulties in voiding.
Treatment of overdosage consists of discontinuation of YUPELRI along with institution of appropriate symptomatic and/or supportive therapy.
DESCRIPTION SECTION
11 DESCRIPTION
YUPELRI is a sterile, clear, colorless, aqueous solution of revefenacin. Revefenacin, the active component of YUPELRI, is an anticholinergic. The chemical name for revefenacin is 1-(2-{4-[(4-carbamoylpiperidin-1-yl)methyl]-N-methylbenzamido}ethyl)piperidin-4-yl N-({1,1’-biphenyl}-2-yl)carbamate; its structural formula is:
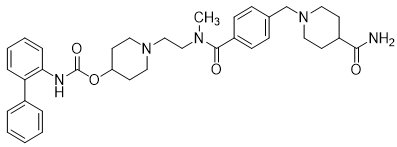
Revefenacin has a molecular weight of 597.76 and its empirical formula is C35H43N5O4. Revefenacin is a white to off-white crystalline powder and is slightly soluble in water.
YUPELRI is supplied as 3 mL of revefenacin solution packaged in a unit-dose low-density polyethylene vial overwrapped in a foil pouch. Each vial contains 175 mcg of revefenacin in 3 mL of an isotonic, sterile aqueous solution containing citric acid, sodium chloride, sodium citrate, and water for injection at pH 5.0. Hydrochloric acid or sodium hydroxide may be used to adjust the pH.
YUPELRI does not require dilution prior to administration by nebulization. Like all other nebulized treatments, the amount delivered to the lungs will depend on patient factors, the nebulization system used, and compressor performance.
Using the PARI LC® Sprint nebulizer connected to a PARI Trek® S compressor under in vitro conditions, the mean delivered dose from the mouthpiece was approximately 62 mcg (35% of label claim), at a mean flow rate of 4 LPM. The mean nebulization time was 8 minutes. YUPELRI should only be administered via a standard jet nebulizer connected to an air compressor with an adequate airflow, and equipped with a mouthpiece.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Revefenacin is a long-acting muscarinic antagonist, which is often referred to as an anticholinergic. It has similar affinity to the subtypes of muscarinic receptors M1 to M5. In the airways, it exhibits pharmacological effects through inhibition of M3 receptor at the smooth muscle leading to bronchodilation. The competitive and reversible nature of antagonism was shown with human and animal origin receptors and isolated organ preparations. In preclinical in vitro as well as in vivo models, prevention of methacholine- and acetylcholine-induced bronchoconstrictive effects was dose-dependent and lasted longer than 24 hours. The clinical relevance of these findings is unknown. The bronchodilation following inhalation of revefenacin is predominantly a site-specific effect.
12.2 Pharmacodynamics
Cardiac Electrophysiology
QTc interval prolongation was studied in a randomized, double-blind, placebo- and positive‑controlled, single dose, crossover trial in 48 healthy subjects. Following a single dose of revefenacin 700 mcg (4 times the recommended dosage), no effects on prolongation of QTc interval were observed.
12.3 Pharmacokinetics
Revefenacin pharmacokinetic parameters are presented as the mean [standard deviation (SD)] unless otherwise specified. Following repeat dosing of inhaled YUPELRI, steady-state was achieved within 7 days with <1.6‑fold accumulation. Revefenacin exposure (Cmax and AUC) in COPD patients is approximately 60% lower as compared to healthy subjects. Exposure (Cmax and AUC) of the active metabolite in COPD patients is approximately 2-fold higher as compared to healthy subjects. Revefenacin Cmax was 0.16 ng/mL (0.11) and AUC was 0.22 ng·hr/mL (0.20) at steady-state after inhaled YUPELRI 175 mcg dose in COPD patients. Cmax of the active metabolite was 0.20 ng/mL (0.13) and AUC was 0.69 ng·hr/mL (0.53) at steady-state after inhaled YUPELRI 175 mcg dose in COPD patients.
Revefenacin and its active metabolite exposure increased in a slightly greater than dose proportional manner with increasing revefenacin dose. After single or multiple once-daily dosing of YUPELRI, both AUC and Cmax of revefenacin and its active metabolite increased by approximately 11-fold over the 88 to 700 mcg (8‑fold) dose range.
Absorption
Following inhaled administration of YUPELRI in healthy subjects or COPD patients, Cmax of revefenacin and its active metabolite occurred at the first postdose sampling time which ranged from 14 to 41 minutes after start of nebulization. The absolute bioavailability following an oral dose of revefenacin is low (<3%).
Distribution
Following intravenous administration to healthy subjects, the mean steady- state volume of distribution of revefenacin was 218 L suggesting extensive distribution to tissues. In vitro protein binding of revefenacin and its active metabolite in human plasma was on average 71% and 42%, respectively.
Elimination
The terminal half-life of revefenacin and its active metabolite after once- daily dosing of YUPELRI in COPD patients is 22 to 70 hours.
Metabolism
In vitro and in vivo data showed that revefenacin is primarily metabolized via hydrolysis of the primary amide to a carboxylic acid forming its major active metabolite. Following inhaled administration of YUPELRI in COPD patients, conversion to its active metabolite occurred rapidly, and plasma exposures of the active metabolite exceeded those of revefenacin by approximately 4- to 6-fold (based on AUC). The active metabolite is formed by hepatic metabolism and possesses activity at target muscarinic receptors that is lower (approximately one-third to one-tenth) than that of revefenacin. It could potentially contribute to systemic antimuscarinic effects at therapeutic doses.
Excretion
Following administration of a single intravenous dose of radiolabeled revefenacin to healthy male subjects, approximately 54% of total radioactivity was recovered in the feces and 27% was excreted in the urine. Approximately 19% of the administered radioactive dose was recovered in the feces as the active metabolite. Following administration of a single radiolabeled oral dose of revefenacin, 88% of total radioactivity was recovered in the feces and <5% was present in urine, suggesting low oral absorption. There was minimal renal excretion (<1%) of revefenacin and its active metabolite following inhaled administration of YUPELRI in COPD patients.
Specific Populations
Population pharmacokinetic analysis showed no evidence of a clinically significant effect of age (44 to 79 years), gender (59% male), smoking status (42% current smoker), or weight (46 to 155 kg) on systemic exposure of revefenacin and its active metabolite.
Patients with Hepatic Impairment
The pharmacokinetics of YUPELRI was evaluated in subjects with moderate hepatic impairment (Child-Pugh score of 7-9). There was no increase in Cmax of revefenacin and 1.5-fold increase in Cmax of the active metabolite. There was 1.2-fold increase in AUC of revefenacin and up to 4.7-fold increase in AUC of the active metabolite. YUPELRI has not been evaluated in subjects with severe hepatic impairment.
Patients with Renal Impairment
The pharmacokinetics of YUPELRI was evaluated in subjects with severe renal impairment (CrCl <30 mL/min). There was 1.5-fold increase in Cmax of revefenacin and up to 2-fold increase in Cmax of the active metabolite. There was up to 2.3‑fold increase in AUCinf of revefenacin; the active metabolite exposure (AUCinf) was increased by up to 2.5-fold. YUPELRI has not been evaluated in subjects with end-stage renal disease.
Drug Interaction Studies
Revefenacin and Cytochrome P450
Neither revefenacin nor its active metabolite inhibits the following cytochrome P450 isoforms: CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, and CYP3A4/5. Neither revefenacin nor its active metabolite induces CYP1A2, CYP2B6, and CYP3A4/5.
Revefenacin and Efflux Transporters
Revefenacin is a substrate of P-gp and BCRP. Neither revefenacin nor its active metabolite is an inhibitor of these efflux transporters.
Revefenacin and Uptake Transporters
The active metabolite of revefenacin is a substrate of OATP1B1 and OATP1B3. Neither revefenacin nor its active metabolite is an inhibitor of the uptake transporters OATP1B1, OATP1B3, OAT1, OAT3, or OCT2.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Two-year inhalation studies in Sprague-Dawley rats and CD1 mice were conducted to assess the carcinogenic potential of revefenacin. No evidence of tumorigenicity was observed in male and female rats at inhaled doses up to 338 mcg/kg/day (approximately 35 times the MRHD based upon summed AUCs for revefenacin and its active metabolite). No evidence of tumorigenicity was observed in male and female mice at inhaled doses up to 326 mcg/kg/day (approximately 40 times the MRHD based on summed AUCs for revefenacin and its active metabolite).
Revefenacin and its active metabolite were negative for mutagenicity in the Ames test for bacterial gene mutation. Revefenacin was negative for genotoxicity in the in vitro mouse lymphoma assay and in vivo rat bone marrow micronucleus assay.
There were no effects on male or female fertility and reproductive performance in rats at subcutaneous revefenacin doses up to 500 mcg/kg/day (approximately 30 times the MRHD on an mg/m2 basis for revefenacin).
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
The safety and efficacy of YUPELRI 175 mcg once daily were evaluated in two dose‑ranging trials, two replicate 12-week, Phase 3 confirmatory clinical trials, and a 52-week safety trial. The efficacy of YUPELRI is primarily based on the two replicate 12-week, Phase 3 placebo-controlled trials in 1,229 subjects with COPD.
14.1 Dose-Ranging Trials
Dose selection for YUPELRI was supported by a 28-day, randomized, double- blind, placebo-controlled, parallel-group trial of 355 subjects diagnosed with moderate to severe COPD, which was conducted to evaluate four doses of YUPELRI. YUPELRI 44, 88, 175, and 350 mcg, or matching placebo were taken once daily in the morning via a standard jet nebulizer (PARI LC® Sprint Reusable Nebulizer) and evaluated using the primary efficacy endpoint of change from baseline in trough (predose) FEV1 measured on Day 29. The LS mean differences in change from baseline in trough FEV1 compared to placebo for the 44 mcg, 88 mcg, 175 mcg, and 350 mcg once-daily doses were 52 mL [95% CI: -17.3, 121.0], 187 mL [95% CI: 118.8, 256.1], 167 mL [95% CI: 97.3, 236.0], and 171 mL [95% CI: 101.9, 239.3], respectively.
Evaluations of the dosing interval by comparing once- and twice-daily dosing of YUPELRI in a 7-day, randomized, double-blind, placebo-controlled, crossover trial in 64 patients supported selection of the once-daily dosing interval for further evaluation in the confirmatory COPD trials.
The dose-ranging results supported the evaluation of two doses of YUPELRI, 88 mcg and 175 mcg once daily, in the confirmatory COPD trials.
14.2 Confirmatory Trials
The clinical development program for YUPELRI included two 12-week, randomized, double-blind, placebo-controlled, multiple-dose, parallel-group, confirmatory trials in subjects with moderate to very severe COPD designed to evaluate the efficacy of once-daily YUPELRI’s effect on lung function (Trial 1: NCT02459080 and Trial 2: NCT02512510). To be enrolled, subjects needed to be 40 years of age or older, have a clinical diagnosis of COPD, a history of smoking greater than or equal to 10 pack-years, moderate to very severe COPD (post‑ipratropium FEV1 less than or equal to 80% of predicted normal values but at least 700 mL), and an FEV1/FVC ratio of 0.7 or less. Trials 1 and 2 included 1,229 subjects of which 395 received the 175 mcg dose administered via a standard jet nebulizer (PARI LC® Sprint Reusable Nebulizer). The study population had a mean age of 64 years (range: 41 to 88) and mean smoking history of 53 pack- years, with 48% identified as current smokers. At screening, the mean post- bronchodilator percent predicted FEV1 was 55% (range: 10% to 90%), and the post-bronchodilator FEV1/FVC ratio was 0.54 (range: 0.3 to 0.7). In addition, of the subjects enrolled, 37% were taking LABA or ICS/LABA therapy at study entry and remained on this concomitant therapy throughout the study.
Trials 1 and 2 evaluated YUPELRI 175 mcg once daily and placebo once daily. The primary endpoint was change from baseline in trough (predose) FEV1 at Day 85. In both trials, YUPELRI 175 mcg demonstrated significant improvement in lung function (mean change from baseline in trough (predose) FEV1) compared to placebo.
Table 2 presents the results from Trial 1 and Trial 2. The change from baseline in trough FEV1 over time from Trial 1 is depicted in Figure 1.
Table 2: LS Mean Change from Baseline in Trough FEV1 (mL) on Day 85 (ITT)|
Trial 1 |
Trial 2 | |||
|---|---|---|---|---|
|
LS – Least Square, SE – Standard Error | ||||
| ||||
|
Placebo (N = 209) |
YUPELRI |
Placebo (N = 208) |
YUPELRI | |
|
n* |
191 |
189 |
187 |
181 |
|
LS Mean (SE) |
-19 (16.1) |
127 (15.4) |
-45 (18.8) |
102 (18.5) |
|
LS Mean Difference (SE) from Placebo |
-- |
146 (21.6) |
-- |
147 (25.5) |
|
95% CI for LS Mean Difference from Placebo |
-- |
(103.7, 188.8) |
-- |
(97.0, 197.1) |
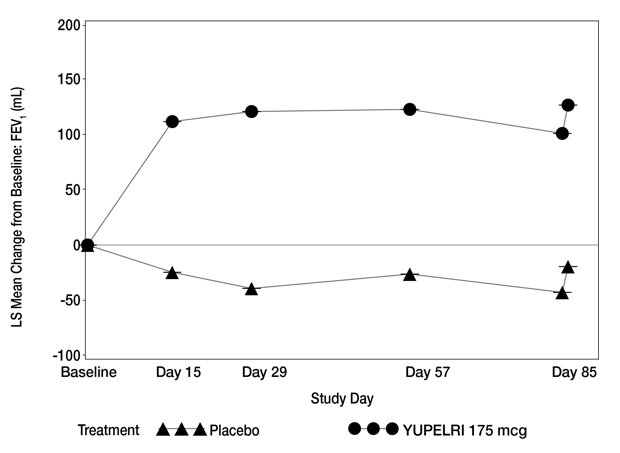
Figure 1: LS Mean Change from Baseline in Trough FEV1 (mL) over 12 Weeks (Trial 1)
In Trial 1, serial spirometry over 24 hours was performed in a subset of patients (n=44 placebo, n=45 YUPELRI 175 mcg) on Day 84. In Trial 2, similar testing was also performed (n=39 placebo, n=44 YUPELRI 175 mcg). That data for Trial 1 is shown in Figure 2.
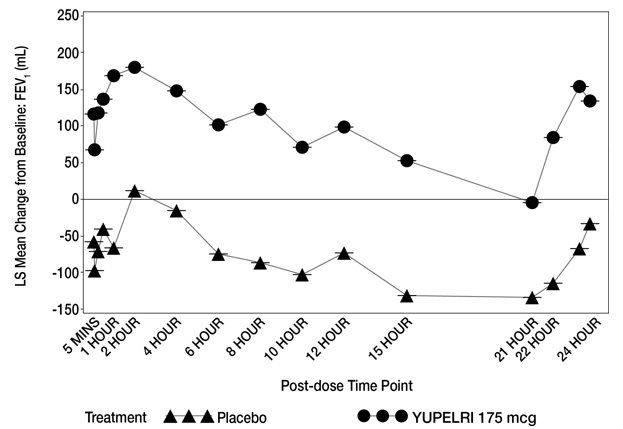
Figure 2: LS Mean Change from Baseline FEV1 (mL) over 24 Hours Day 84 (Trial 1 subset)
Peak FEV1 was defined as the highest postdose FEV1 within the first 2 hours after dosing on Day 1. The mean peak FEV1 improvement on Day 1 relative to placebo was 133 mL and 129 mL in Trials 1 and 2, respectively.
The St. Georges Respiratory Questionnaire (SGRQ) was assessed in Trials 1 and 2. In Trial 1, the SGRQ responder rate (defined as an improvement in score of 4 or more as threshold) for the YUPELRI treatment arm on Day 85 was 49% compared to 34% for placebo [Odds Ratio: 2.11; 95% CI: 1.14, 3.92]. In Trial 2, the SGRQ responder rate for the YUPELRI treatment arm was 45% compared to 39% for placebo [Odds Ratio: 1.31; 95% CI: 0.72, 2.38].
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Inhalation solution: YUPELRI is supplied as a 175 mcg/3 mL sterile, clear, colorless, aqueous solution in unit-dose low‑density polyethylene vials. Each vial is overwrapped in a foil pouch and supplied in cartons containing either 30 individually pouched unit‑dose vials (NDC 49502-806-93) or 7 individually pouched unit-dose vials (NDC 49502-806-77).
Storage and Handling
•
Store YUPELRI in the protective foil pouch.
•
Store at room temperature from 68°F to 77°F (20°C to 25°C); excursions permitted from 59°F to 86°F (15°C to 30°C) [See USP Controlled Room Temperature]. Protect from direct sunlight and excessive heat.
The YUPELRI solution unit-dose vial should only be removed from the foil pouch and opened IMMEDIATELY BEFORE USE. The vial and any residual content should be discarded after use.
•
Discard any solution that is not clear and colorless.
•
YUPELRI should only be administered via a standard jet nebulizer connected to an air compressor with an adequate airflow, and equipped with a mouthpiece.
•
Do not swallow or inject YUPELRI.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Not for Acute Symptoms
Inform patients that YUPELRI is not meant to relieve acute symptoms of COPD and extra doses should not be used for that purpose. Advise patients to treat acute symptoms with an inhaled, short-acting beta2-agonist such as albuterol [see Warnings and Precautions (5.1)]. Provide patients with such medicine and instruct them in how it should be used.
Instruct patients to seek medical attention immediately if they experience any of the following:
•
Decreasing effectiveness of inhaled, short-acting beta2-agonists
•
Need for more inhalations than usual of inhaled, short-acting beta2-agonists
•
Significant decrease in lung function as outlined by the physician
Tell patients they should not stop therapy with YUPELRI without healthcare provider guidance since symptoms may recur after discontinuation.
Paradoxical Bronchospasm
As with other inhaled medicines, YUPELRI can cause paradoxical bronchospasm. If paradoxical bronchospasm occurs, instruct patients to discontinue YUPELRI [see Warnings and Precautions (5.2)].
Worsening of Narrow-Angle Glaucoma
Instruct patients to be alert for signs and symptoms of acute narrow-angle glaucoma (e.g. eye pain or discomfort, blurred vision, visual halos, or colored images in association with red eyes from conjunctival congestion and corneal edema). Instruct patients to consult a healthcare provider immediately if any of these signs or symptoms develops [see Warnings and Precautions (5.3)].
Worsening of Urinary Retention
Instruct patients to be alert for signs and symptoms of urinary retention (e.g. difficulty passing urine, painful urination). Instruct patients to consult a healthcare provider immediately if any of these signs or symptoms develops [see Warnings and Precautions (5.4)].
Instructions for Administering YUPELRI
It is important for patients to understand how to correctly administer YUPELRI using a standard jet nebulizer [see Instructions for Use]. Instruct patients that YUPELRI should only be administered via a standard jet nebulizer. Patients should be instructed not to inject or swallow the YUPELRI solution. Patients should be instructed not to mix other medications with YUPELRI.
Patients should not inhale more than one dose at any one time. The daily dosage of YUPELRI should not exceed one unit-dose vial. Inform patients to use the contents of one vial of YUPELRI orally inhaled daily at the same time every day. Patients should throw the plastic dispensing vials away immediately after use. Due to their small size, the vials pose a danger of choking to young children.
The brands listed are trademarks of their respective owners.
Licensed from:
Theravance Biopharma Ireland Limited
Manufactured for:
Mylan Specialty L.P.
Morgantown, WV 26505 USA
Made in USA
© 2021 Viatris Inc.
YUPELRI® is a registered trademark of Mylan Specialty L.P., a Viatris Company.
Patented. See YUPELRI.com/ patents
SPL PATIENT PACKAGE INSERT SECTION
PATIENT INFORMATION
|
YUPELRI**®**** (you-PELL-ree)** (revefenacin) inhalation solution, for oral inhalation | |
|
Important: For oral inhalation only. Do not swallow or inject YUPELRI. | |
|
What is YUPELRI? • • • • • | |
|
Do not use YUPELRI if youhave had an allergic reaction to revefenacin or any of the ingredients in YUPELRI. Ask your healthcare provider if you are not sure. See the end of this Patient Information leaflet for a complete list of ingredients in YUPELRI. | |
|
Before using YUPELRI, tell your healthcare provider about all your medical conditions, including if you: • • • • • • **Tell your healthcare provider about all the medicines you take,**including prescription and over-the-counter medicines, vitamins, and herbal supplements. YUPELRI and certain other medicines may interact with each other. This may cause serious side effects. Especially tell your healthcare provider if you take: • • Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist each time you get a new medicine. | |
|
How should I use YUPELRI? Read the step-by-step instructions for using YUPELRI at the end of this Patient Information leaflet. • • • • • • • • • • • • • • • | |
|
What are the possible side effects with YUPELRI? YUPELRI can cause serious side effects, including: • • | |
|
o o o |
o o |
• | |
|
o o |
o o |
• | |
|
o o o |
o o |
|
• | |
|
o o o |
o o |
|
These are not all the possible side effects of YUPELRI. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1‑800-FDA-1088. | |
|
How should I store YUPELRI? • • • • • • • | |
|
General Information about the safe and effective use of YUPELRI. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use YUPELRI for a condition for which it was not prescribed. Do not give YUPELRI to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about YUPELRI that is written for health professionals. | |
|
What are the ingredients in YUPELRI? Active ingredient: revefenacin Inactive ingredients: sodium chloride, citric acid, sodium citrate, and water for injection. Hydrochloric acid or sodium hydroxide may be used to adjust the pH. Licensed from: Manufactured for: Made in USA © 2021 Viatris Inc. YUPELRI® is a registered trademark of Mylan Specialty L.P., a Viatris Company. Patented. See YUPELRI.com/patents For more information about YUPELRI, go to www.YUPELRI.com or call 1-877-446-3679 (1-877-4-INFO-RX). |
This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: 11/2021
|
INSTRUCTIONS FOR USE |
|
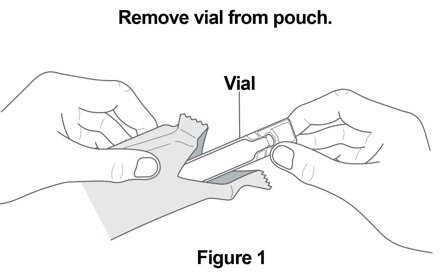
YUPELRI is used only in a standard jet nebulizer machine with a mouthpiece connected to an air compressor. Make sure you know how to use your nebulizer machine before you use it to breathe in YUPELRI or other medicines. Important Information: • • • Using YUPELRI: Read the following Steps before using YUPELRI. If you have any questions, ask your healthcare provider or pharmacist. Step 1.Open Pouch: Open the foil pouch by tearing along the seam of the pouch. Remove the vial of YUPELRI from the foil pouch (Figure 1). |
|
|
|
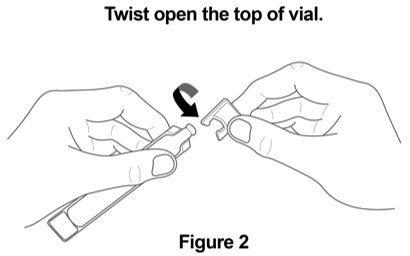
Step 2. Open Vial: Carefully twist open the top of the vial and use it right away (Figure 2). |
|
|
|
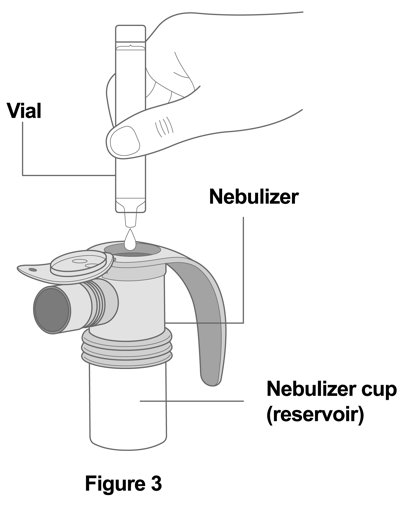
Step 3. Add Medicine: Squeeze all of the medicine from the vial into the nebulizer cup (reservoir) (Figure 3).
|
|
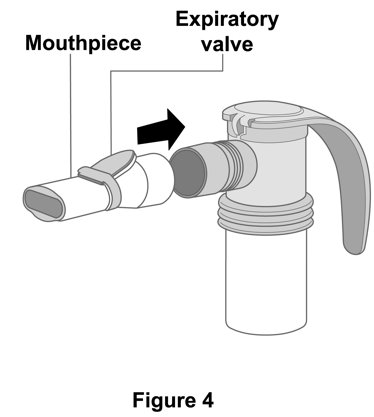
**Step 4. Attach Mouthpiece:**Connect the mouthpiece to the nebulizer cup (reservoir) with the expiratory valve facing up (Figure 4). |
|
|
|
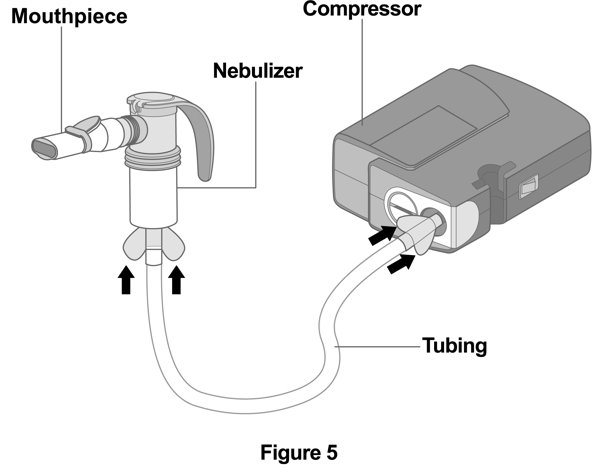
Step 5. Connect the Nebulizer to the Compressor: Firmly insert one end of the tubing to the compressor. Insert the other end of the tubing to the bottom of the nebulizer cup (reservoir) (Figure 5). |
|
|
|
Step 6. Prepare for Treatment: Sit in a comfortable, upright position. Place the mouthpiece in your mouth and close your lips around the mouthpiece. Step 7. Begin Treatment:Turnon the compressor to begin your treatment (Figure 6). |
|
|
|
Step 8. Breathe in Medicine: Breathe as calmly, deeply, and evenly as possible until no more mist is seen in the nebulizer reservoir. Your treatment will take about 8 minutes. When you do not see any mist in the nebulizer, your treatment is finished. Turn the compressoroff. **Step 9. Clean Nebulizer:**Clean and store your nebulizer. See the manufacturer’s instructions that come with your nebulizer for how to clean and store your nebulizer. |
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Licensed from:
Theravance Biopharma Ireland Limited
Manufactured for:
Mylan Specialty L.P.
Morgantown, WV 26505 USA
Made in USA
© 2021 Viatris Inc.
YUPELRI® is a registered trademark of Mylan Specialty L.P., a Viatris Company.
Patented. See YUPELRI.com/patents
Revised: 5/2022
MS:RVFN:R4
RPINXXXX