Gentle Laxative
Equate 44-607-Gentle
1c27244f-f0e9-43bf-a5fa-f594952914ca
HUMAN OTC DRUG LABEL
Sep 15, 2025
Wal-Mart Stores Inc
DUNS: 051957769
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Bisacodyl
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (33)
Drug Labeling Information
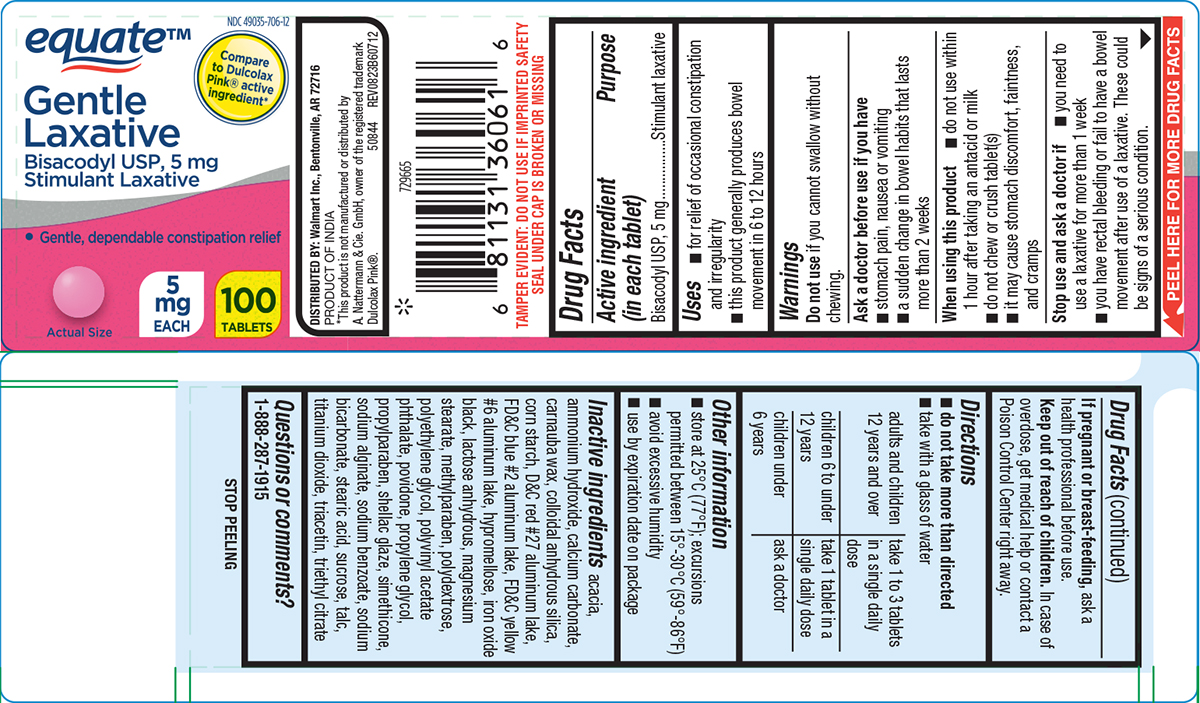
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Principal display panel
equate**™**
NDC 49035-706-12
Compare
to Dulcolax
Pink® active
ingredient*
Gentle
** Laxative**
Bisacodyl USP, 5 mg
Stimulant Laxative
• Gentle, dependable constipation relief
Actual Size
5
** mg**
** EACH**
100
** TABLETS**
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY
** SEAL UNDER CAP IS BROKEN OR MISSING**
DISTRIBUTED BY: Walmart Inc., Bentonville, AR 72716
** PRODUCT OF INDIA**
*This product is not manufactured or distributed by
A. Nattermann & Cie. GmbH, owner of the registered trademark
Dulcolax Pink®. 50844 REV0823B60712
Equate 44-607
INDICATIONS & USAGE SECTION
Uses
- for relief of occasional constipation and irregularity
- this product generally produces bowel movement in 6 to 12 hours
OTC - ACTIVE INGREDIENT SECTION
Active ingredient (in each tablet)
Bisacodyl USP, 5 mg
OTC - PURPOSE SECTION
Purpose
Stimulant laxative
WARNINGS SECTION
Warnings
Do not use
if you cannot swallow without chewing.
Ask a doctor before use if you have
-
stomach pain, nausea or vomiting
-
a sudden change in bowel habits that lasts more than 2 weeks
When using this product
- do not use within 1 hour after taking an antacid or milk
- do not chew or crush tablet(s)
- it may cause stomach discomfort, faintness, and cramps
Stop use and ask a doctor if
-
you need to use a laxative for more than 1 week
-
you have rectal bleeding or fail to have a bowel movement after use of a laxative. These could be signs of a serious condition.
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
DOSAGE & ADMINISTRATION SECTION
Directions
*do not take more than directed
- take with a glass of water
|
adults and children |
take 1 to 3 tablets |
|
children 6 to under |
take 1 tablet in a |
|
children under 6 years |
ask a doctor |
STORAGE AND HANDLING SECTION
Other information
-
store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F)
-
avoid excessive humidity
-
use by expiration date on package
INACTIVE INGREDIENT SECTION
Inactive ingredients
acacia, ammonium hydroxide, calcium carbonate, carnauba wax, colloidal anhydrous silica, corn starch, D&C red #27 aluminum lake, FD&C blue #2 aluminum lake, FD&C yellow #6 aluminum lake, hypromellose, iron oxide black, lactose anhydrous, magnesium stearate, methylparaben, polydextrose, polyethylene glycol, polyvinyl acetate phthalate, povidone, propylene glycol, propylparaben, shellac glaze, simethicone, sodium alginate, sodium benzoate, sodium bicarbonate, stearic acid, sucrose, talc, titanium dioxide, triacetin, triethyl citrate
OTC - QUESTIONS SECTION
Questions or comments?
1-888-287-1915
