E2 hand wash
a40afa46-a07d-f864-e053-2a95a90aef8a
HUMAN OTC DRUG LABEL
May 1, 2025
Horizon Tool Inc.
DUNS: 602012460
Products 10
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
E2 Sanitizing Hand Soap
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
ALCOHOL
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
ALCOHOL
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (4)
E2 Sanitizing Hand Soap
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
ALCOHOL
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (4)
ALCOHOL
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (4)
ALCOHOL
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (4)
ALOCOHL
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (4)
ALCOHOL
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
ALCOHOL
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (4)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Package Label - Principal Display Panel
3785.5 mL NDC: 74683-6400-1
3785.4 mL NDC: 74683-6300-1 
3785.41 mL NDC: 74683-2001-2

473 mL NDC: 74683-2001-1

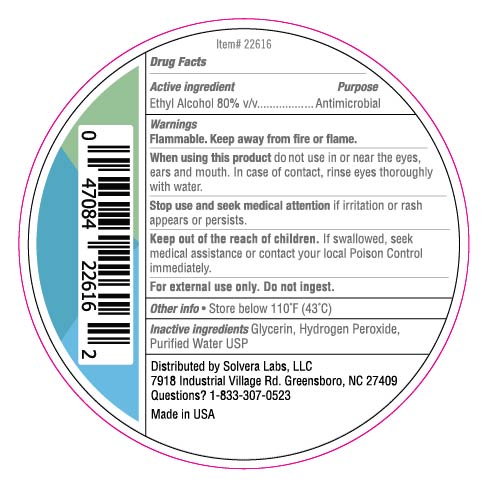
118 mL NDC: 74683-5001-4

250 mL NDC: 74683-5001-3

59 mL NDC: 74683-5001-2

473 mL NDC: 74683-5001-1

250 mL NDC: 74683-1002-1

3785.4 mL NDC: 74683-6200-3

250 mL NDC: 7463-6200-2

3785.4 mL NDC: 74683-6200-1

3785.4 mL NDC: 74683-6000-1 
59 mL NDC: 74683-2100-1 
946.35 mL NDC: 74683-3001-1 
262 mL NDC: 74683-2500-1 
1892.71 mL NDC: 74683-5000-9 
208.2 L NDC: 74683-5000-8 
946.35 mL NDC: 74683-5000-7 
946.35 mL NDC: 74683-5000-7 
473 mL NDC: 74683-5000-6 
3785.41 mL NDC: 74683-5000-5 
3785.41 mL NDC: 74683-5000-4 
354.8 mL NDC: 74683-5000-3 
236 mL NDC: 74683-5000-2 
118 mL NDC: 74683-5000-1 
118 mL NDC: 74683-4000-1

3 mL NDC 74683-3000-8

3 mL NDC 74683-3000-8

3 mL NDC 74683-3000-9

3 mL NDC 74683-3000-9

3785.41 mL NDC 74683-3000-7

3785.41 mL NDC 74683-3000-6

3785.41 mL NDC 74683-3000-5

1892.71 mL NDC 74683-3000-4

946.35 mL NDC 74683-3000-3

946.35 mL NDC 74683-3000-3

118 mL NDC 74683-3000-2

59 mL NDC 74683-3000-1

59 mL NDC 74683-3000-1
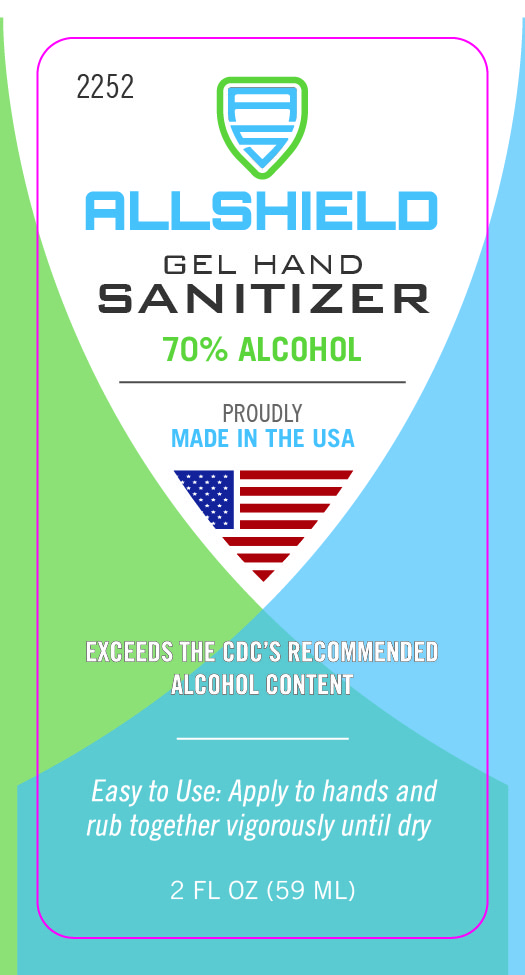
473 mL NDC 74683-1000-9

236 mL NDC 74683-1000-8 
059 mL NDC: 74683-1000-6
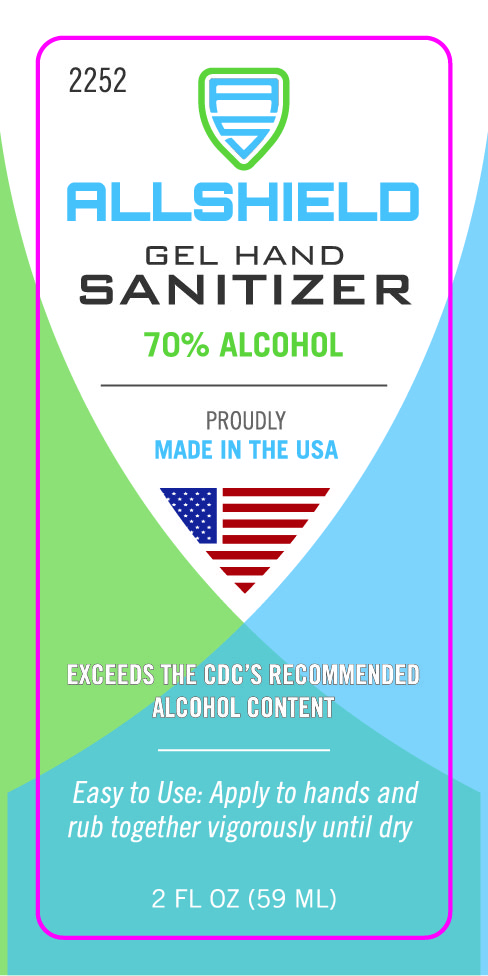
059 mL NDC: 74683-1000-6

74683-1000-7

236 mL NDC: 74683-2000-1  473
mL NDC: 74683-1000-2
473
mL NDC: 74683-1000-2

946.35 mL NDC: 74683-1000-3  946.35
mL NDC: 74683-1000-3
946.35
mL NDC: 74683-1000-3

1892.71 mL NDC: 74683-1000-4
 3785.41
mL NDC: 74683-1000-5
3785.41
mL NDC: 74683-1000-5
 473
mL NDC: 74683-2000-1
473
mL NDC: 74683-2000-1
 473
mL NDC: 74683-2000-1
473
mL NDC: 74683-2000-1
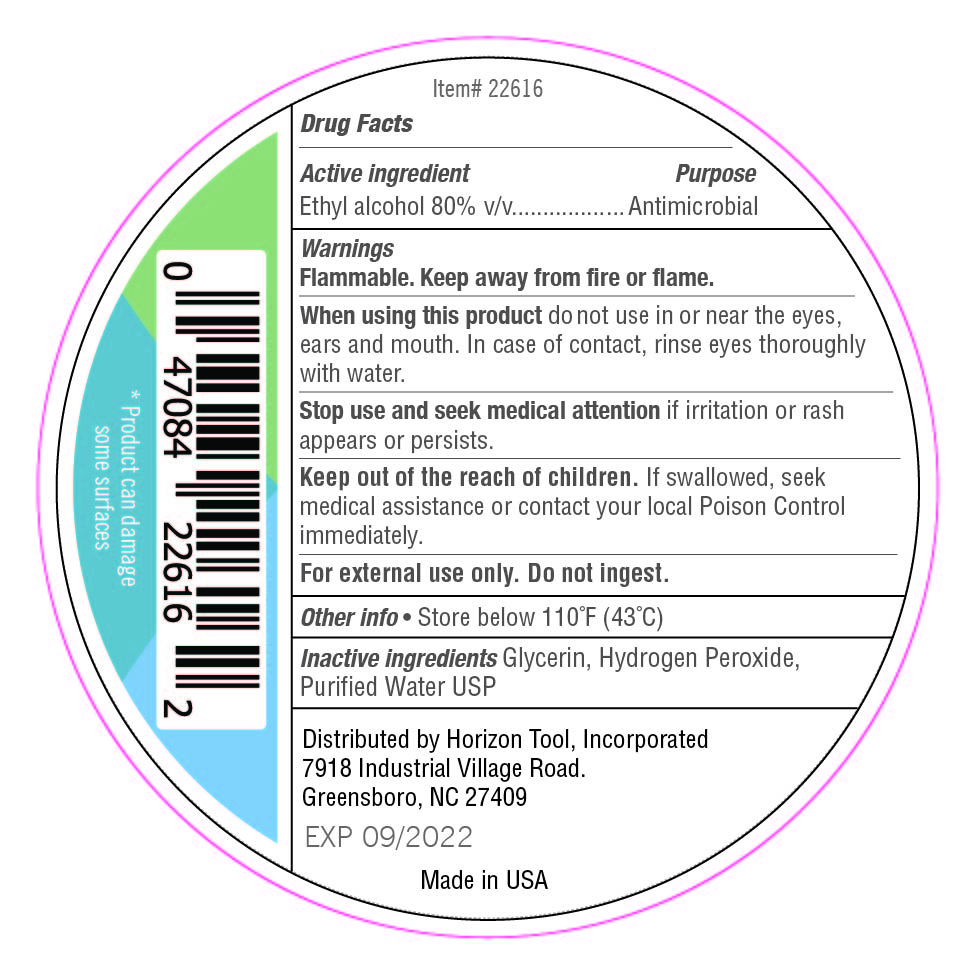 946.35
mL NDC: 74683-2000-2
946.35
mL NDC: 74683-2000-2
 946.35
mL NDC: 74683-2000-2
946.35
mL NDC: 74683-2000-2
 1892.71
mL NDC: 74683-2000-3
1892.71
mL NDC: 74683-2000-3
 3785.41
mL NDC: 74683-2000-4
3785.41
mL NDC: 74683-2000-4

