EMPLICITI
These highlights do not include all the information needed to use EMPLICITI safely and effectively. See full prescribing information for EMPLICITI. EMPLICITI (elotuzumab) for injection, for intravenous useInitial U.S. Approval: 2015
80686b7e-f6f4-4154-b5c0-c846425e2d91
HUMAN PRESCRIPTION DRUG LABEL
Mar 22, 2022
E.R. Squibb & Sons, L.L.C.
DUNS: 011550092
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
elotuzumab
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
elotuzumab
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
Drug Labeling Information
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Infusion Reactions
EMPLICITI can cause infusion reactions. Infusion reactions were reported in 10% of patients treated with EMPLICITI in the ELOQUENT-2 trial and 3.3% in the ELOQUENT-3 trial. In the ELOQUENT-2 trial, all reports of infusion reaction were Grade 3 or lower. In the ELOQUENT-2 trial, Grade 3 infusion reactions occurred in 1% of patients. The most common symptoms of an infusion reaction included fever, chills, and hypertension. Bradycardia and hypotension also developed during infusions.
In the ELOQUENT-2 trial, 5% of patients required interruption of the administration of EMPLICITI for a median of 25 minutes due to infusion reactions, and 1% of patients discontinued due to infusion reactions. Of the patients who experienced an infusion reaction, 70% (23/33) had them during the first dose.
In the ELOQUENT-3 trial, the only infusion reaction symptom was chest discomfort (2%), which was Grade 1. All patients in the ELOQUENT-3 trial who experienced an infusion reaction had them during the first treatment cycle.
Administer premedication consisting of dexamethasone, antihistamines (H1 and H2 blockers) and acetaminophen prior to EMPLICITI infusion [see Dosage and Administration (2.3)].
Interrupt EMPLICITI infusion for Grade 2 or higher infusion reactions and institute appropriate medical management [see Dosage and Administration (2.4)].
5.2 Infections
In the ELOQUENT-2 trial (N=635), infections were reported in 81% of patients in EMPLICITI combined with lenalidomide and dexamethasone (E-Ld) arm and 74% in lenalidomide and dexamethasone (Ld). In the ELOQUENT-3 trial (N=115), infections were reported in 65% of patients in both EMPLICITI combined with pomalidomide and dexamethasone (E-Pd) arm and in pomalidomide and dexamethasone (Pd) arm. In the ELOQUENT-2 trial, Grade 3 to 4 infections were noted in 28% and 24% of E-Ld- and Ld-treated patients and in the ELOQUENT-3 trial, 13% and 22% of E-Pd- and Pd-treated patients, respectively. Discontinuations due to infections occurred in 3.5% of E-Ld-treated and 4.1% of Ld-treated patients in the ELOQUENT-2 trial and 7% of E-Pd-treated and 5% of Pd-treated patients in the ELOQUENT-3 trial. Fatal infections were reported in 2.5% and 2.2% of E-Ld- and Ld-treated patients in the ELOQUENT-2 trial and 5% and 3.6% of E-Pd- and Pd-treated patients in the ELOQUENT-3 trial.
Opportunistic infections were reported in 22% of patients in the E-Ld arm and 13% of patients in the Ld arm in the ELOQUENT-2 trial and 10% of patients in the E-Pd arm and 9% of patients in the Pd arm in the ELOQUENT-3 trial. In the ELOQUENT-2 trial, fungal infections occurred in 10% of patients in the E-Ld arm and 5% of patients in the Ld arm. Herpes zoster was reported in 14% of patients treated with E-Ld and 7% of patients treated with Ld in the ELOQUENT-2 trial and 5% of patients treated with E-Pd and 1.8% of patients treated with Pd in the ELOQUENT-3 trial.
Monitor patients for development of infections and treat promptly.
5.3 Second Primary Malignancies
In the ELOQUENT-2 trial (N=635), invasive second primary malignancies (SPM) have been observed in 9% of patients treated with E-Ld and 6% of patients treated with Ld and in the ELOQUENT-3 trial (N=115) in 1.8% of patients treated with Pd and in none of the patients treated with E-Pd. In the ELOQUENT-2 trial, the rate of hematologic malignancies were the same between E-Ld and Ld treatment arms (1.6%). Solid tumors were reported in 3.5% and 2.2% of E-Ld- and Ld-treated patients, respectively. Skin cancer was reported in 4.4% and 2.8% of patients treated with E-Ld and Ld, respectively.
Monitor patients for the development of second primary malignancies.
5.4 Hepatotoxicity
In the ELOQUENT-2 trial (N=635), elevations in liver enzymes (aspartate transaminase/alanine transaminase [AST/ALT] greater than 3 times the upper limit, total bilirubin greater than 2 times the upper limit, and alkaline phosphatase less than 2 times the upper limit) consistent with hepatotoxicity were reported in 2.5% and 0.6% of E-Ld- and Ld-treated patients. Two patients experiencing hepatotoxicity were not able to continue treatment; however, 6 out of 8 patients had resolution and were able to continue treatment. Monitor liver enzymes periodically. Stop EMPLICITI upon Grade 3 or higher elevation of liver enzymes. After return to baseline values, continuation of treatment may be considered.
5.5 Interference with Determination of Complete Response
EMPLICITI is a humanized IgG kappa monoclonal antibody that can be detected on both the serum protein electrophoresis (SPEP) and immunofixation (IFE) assays used for the clinical monitoring of endogenous M-protein [see Drug Interactions (7.2)]. This interference can impact the determination of complete response and possibly relapse from complete response in patients with IgG kappa myeloma protein.
•
Infusion reactions: Premedication is required. Interrupt EMPLICITI for Grade 2 or higher and permanently discontinue for severe infusion reaction. (2.3, 2.4, 5.1)
•
Infections: Monitor for fever and other signs of infection and treat promptly. (5.2)
•
Second Primary Malignancies (SPM): Higher incidences of SPM were observed in a controlled clinical trial of patients with multiple myeloma receiving EMPLICITI. (5.3)
•
Hepatotoxicity: Monitor liver function and stop EMPLICITI if hepatotoxicity is suspected. (5.4)
•
Interference with determination of complete response: EMPLICITI can interfere with assays used to monitor M-protein. This interference can impact the determination of complete response. (5.5)
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Drug Interactions
For important drug interactions involving lenalidomide, pomalidomide and dexamethasone, refer to their respective prescribing information.
7.2 Laboratory Test Interference
EMPLICITI may be detected in the SPEP and serum immunofixation assays of myeloma patients and could interfere with correct response classification. A small peak in the early gamma region on SPEP that is IgGƙ on serum immunofixation may potentially be attributed to EMPLICITI, particularly in patients whose endogenous myeloma protein is IgA, IgM, IgD, or lambda light chain restricted. This interference can impact the determination of complete response and possibly relapse from complete response in patients with IgG kappa myeloma protein [see Warnings and Precautions (5.5)].
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on EMPLICITI use in pregnant women to inform a drug associated risk of major birth defects and miscarriage. Animal reproduction studies have not been conducted with elotuzumab.
EMPLICITI is administered in combination with lenalidomide and dexamethasone or pomalidomide and dexamethasone. Lenalidomide and pomalidomide can cause embryo-fetal harm and are contraindicated for use in pregnancy. Refer to the lenalidomide, pomalidomide and dexamethasone prescribing information for additional information. Lenalidomide and pomalidomide are only available through a REMS program.
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
8.2 Lactation
Risk Summary
There are no data on the presence of EMPLICITI in human milk, the effects on the breastfed child, or the effects on milk production. Because of the potential for serious adverse reactions in the breastfed child from elotuzumab administered in combination with lenalidomide and dexamethasone or pomalidomide and dexamethasone, advise lactating women not to breastfeed during treatment with EMPLICITI. Refer to the lenalidomide, pomalidomide and dexamethasone prescribing information for additional information.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Refer to the lenalidomide and pomalidomide labeling for pregnancy testing requirements prior to initiating treatment in females of reproductive potential.
When EMPLICITI is used with lenalidomide or pomalidomide, there is a risk of fetal harm, including severe life-threatening human birth defects associated with lenalidomide and pomalidomide, and the need to follow requirements regarding pregnancy avoidance, including testing.
Contraception
Females
Refer to the lenalidomide and pomalidomide labeling for contraception requirements prior to initiating treatment in females of reproductive potential.
Males
Lenalidomide and pomalidomide are present in the blood and semen of patients receiving the drug. Refer to the lenalidomide and pomalidomide full prescribing information for requirements regarding contraception and the prohibitions against blood and/or sperm donation due to presence and transmission in blood and/or semen and for additional information.
8.4 Pediatric Use
Safety and effectiveness have not been established in pediatric patients.
8.5 Geriatric Use
Of the 646 patients across treatment groups in the ELOQUENT-2 randomized trial designed to evaluate the use of EMPLICITI in combination with lenalidomide and low-dose dexamethasone in multiple myeloma, 57% were 65 years of age or older; the number of patients 65 years or older was similar between treatment groups. No overall differences in efficacy or safety were observed between patients 65 years or older and younger patients (less than 65 years of age).
Of the 117 patients across treatment groups in the ELOQUENT-3 randomized trial designed to evaluate the use of EMPLICITI in combination with pomalidomide and low-dose dexamethasone in multiple myeloma, 62% were 65 years of age or older; the number of patients 65 years or older was similar between treatment groups. This study did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects.
Pregnancy: Embryo-fetal toxicity in combination with lenalidomide and dexamethasone or pomalidomide and dexamethasone. (8.1)
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
ELOQUENT-2 (NCT01239797)
The efficacy and safety of EMPLICITI in combination with lenalidomide and dexamethasone were evaluated in ELOQUENT-2, a randomized, open-label trial in patients with multiple myeloma who had received one to three prior therapies and had documented progression following their most recent therapy.
Eligible patients were randomized in a 1:1 ratio to receive either EMPLICITI in combination with lenalidomide and low-dose dexamethasone or lenalidomide and low-dose dexamethasone. Treatment was administered in 4-week cycles until disease progression or unacceptable toxicity. EMPLICITI 10 mg/kg was administered intravenously each week for the first 2 cycles and every 2 weeks thereafter. Prior to EMPLICITI infusion, dexamethasone was administered as a divided dose: an oral dose of 28 mg and an intravenous dose of 8 mg. In the control group and on weeks without EMPLICITI, dexamethasone 40 mg was administered as a single oral dose weekly. Lenalidomide 25 mg was taken orally once daily for the first 3 weeks of each cycle. Assessment of tumor response was conducted every 4 weeks.
A total of 646 patients were randomized to receive treatment: 321 to EMPLICITI in combination with lenalidomide and low-dose dexamethasone and 325 to lenalidomide and low-dose dexamethasone.
Demographics and baseline disease characteristics were balanced between treatment arms. The median age was 66 years (range, 37-91); 57% of patients were 65 years or older; 60% of patients were male; whites comprised 84% of the study population, Asians 10%, and blacks 4%. The ECOG performance status was 0 in 47%, 1 in 44%, and 2 in 9% of patients, and ISS Stage was I in 43%, II in 32%, and III in 21% of patients. The cytogenetic categories of del 17p and t(4;14) were present in 32% and 9% of patients, respectively. The median number of prior therapies was 2. Thirty-five percent (35%) of patients were refractory (progression during or within 60 days of last therapy) and 65% were relapsed (progression after 60 days of last therapy). Prior therapies included stem cell transplant (55%), bortezomib (70%), melphalan (65%), thalidomide (48%), and lenalidomide (6%).
The efficacy of EMPLICITI was evaluated by progression-free survival (PFS) as assessed by hazard ratio, and overall response rate (ORR) as determined by a blinded Independent Review Committee using the European Group for Blood and Marrow Transplantation (EBMT) response criteria. Efficacy results are shown in Table 12 and Figure 1. The median number of treatment cycles was 19 for the EMPLICITI group and 14 for the comparator arm with a minimum follow-up of two years.
A pre-planned final overall survival (OS) analysis was performed after at least 427 deaths occurred. The minimum follow-up was 70.6 months. The OS results at final analysis reached statistical significance. A significantly longer OS was observed in patients in the E-Ld arm compared to patients in Ld arm, representing an 18% reduction in the risk of death. Efficacy results are presented in Table 12 and Figure 2.
Table 12: ELOQUENT-2 Efficacy Results|
EMPLICITI + |
Lenalidomide/ N=325 | |
|---|---|---|
|
PFS | ||
| ||
|
Hazard Ratio [95% CI] |
0.70 [0.57, 0.85] | |
|
Stratified log-rank test p-value* |
0.0004 | |
|
Median PFS in months [95% CI] |
19.4 [16.6, 22.2] |
14.9 [12.1, 17.2] |
|
Response | ||
|
Overall Response (ORR)† n (%) |
252 (78.5) |
213 (65.5) |
|
p-value‡ |
0.0002 | |
|
Complete Response (CR + sCR)†,§ n (%) |
14 (4.4)¶ |
24 (7.4) |
|
Very Good Partial Response (VGPR)† n (%) |
91 (28.3) |
67 (20.6) |
|
Partial Response (PR)† n (%) |
147 (45.8) |
122 (37.5) |
|
Overall Survival | ||
|
Hazard Ratio [95.4% CI] |
0.82 [0.68, 1.00] | |
|
Stratified log-rank test p-value* |
0.0408 | |
|
Median OS in months [95% CI] |
48.3 [40.3, 51.9] |
39.6 [33.3, 45.3] |
Figure 1: ELOQUENT-2 Progression-Free Survival
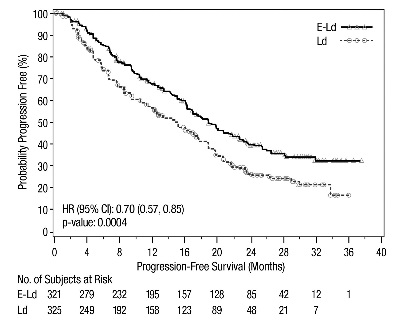
The 1- and 2-year rates of PFS for EMPLICITI in combination with lenalidomide and dexamethasone treatment were 68% and 41%, respectively, compared with 57% and 27%, respectively, for lenalidomide and dexamethasone treatment.
Figure 2: ELOQUENT-2 Overall Survival

ELOQUENT-3 (NCT02654132)
The efficacy and safety of EMPLICITI in combination with pomalidomide and dexamethasone were evaluated in ELOQUENT-3, a randomized, open-label trial in patients with relapsed or refractory multiple myeloma.
Eligible patients were randomized in a 1:1 ratio to receive either EMPLICITI in combination with pomalidomide and low-dose dexamethasone or pomalidomide and low-dose dexamethasone. Treatment was administered in 4-week cycles until disease progression or unacceptable toxicity. EMPLICITI 10 mg/kg was administered intravenously each week for the first 2 cycles and 20 mg/kg every 4 weeks thereafter.
Prior to EMPLICITI infusion, dexamethasone was administered. Dexamethasone was administered on day 1, 8, 15 and 22 of each cycle. On weeks with EMPLICITI infusion, dexamethasone was administered as a divided dose: subjects 75 years or younger, an oral dose of 28 mg and an intravenous dose of 8 mg, and in subjects older than 75 years an oral dose of 8 mg and an intravenous dose of 8 mg. On weeks without an EMPLICITI infusion and in the control group, dexamethasone was administered in subjects 75 years or younger as an oral dose of 40 mg and in subjects older than 75 years as an oral dose of 20 mg dexamethasone was administered orally. Assessment of tumor response was conducted every 4 weeks.
A total of 117 patients were randomized to receive treatment: 60 to EMPLICITI in combination with pomalidomide and low-dose dexamethasone and 57 to pomalidomide and low-dose dexamethasone.
Demographics and baseline disease characteristics were balanced between treatment arms. The median age was 67 years (range, 36-81); 62% of patients were 65 years or older; 57% of patients were male; whites comprised 77% of the study population, Asians 21%, and blacks 1%. The ECOG performance status was 0 in 44%, 1 in 46%, and 2 in 10% of patients, and ISS Stage was I in 50%, II in 38%, and III in 12% of patients. The chromosomal lab abnormalities as determined by FISH of del 17p and t(4;14) were present in 5% and 11% of patients, respectively. The median number of prior therapies was 3. Eighty- seven percent (87%) of patients were refractory to lenalidomide, 80% refractory to a proteasome inhibitor, 70% were refractory to both lenalidomide and a proteasome inhibitor. Prior therapies included stem cell transplant (55%), bortezomib (100%), lenalidomide (99%), cyclophosphamide (66%), melphalan (63%), carfilzomib (21%), and daratumumab (3%).
The efficacy of EMPLICITI was evaluated by progression-free survival (PFS) and overall response rate (ORR) as determined by the investigator. Efficacy results are shown in Table 13 and Figure 3. The median number of treatment cycles was 9 for the EMPLICITI group and 5 for the comparator arm with a minimum follow-up of 9.1 months.
A pre-planned final OS analysis was performed after at least 78 deaths occurred. The minimum follow-up was 45.0 months. A longer OS was observed in patients in the E-Pd arm compared to patients in the Pd arm. Efficacy results are presented in Table 13 and Figure 4.
Table 13: ELOQUENT-3 Efficacy Results|
EMPLICITI + |
Pomalidomide/ N=57 | |
|---|---|---|
| ||
|
PFS | ||
|
Hazard Ratio [95% CI] |
0.54 [0.34, 0.86] | |
|
Stratified log-rank test p-value* |
0.0078 | |
|
Median PFS in months [95% CI] |
10.25 [5.59, NE] |
4.67 [2.83, 7.16] |
|
Response | ||
|
Overall Response (ORR)† n (%) |
32 (53.3) |
15 (26.3) |
|
p-value‡ |
0.0029 | |
|
Complete Response (CR + sCR)†,§ n (%) |
5 (8.3)¶ |
1 (1.8) |
|
Very Good Partial Response (VGPR)† n (%) |
7 (11.7) |
4 (7.0) |
|
Partial Response (PR)† n (%) |
20 (33.3) |
10 (17.5) |
|
Overall Survival | ||
|
Number of death events |
37 (61.7) |
41 (74.5) |
|
Hazard Ratio [95% CI] |
0.59 [0.37, 0.93] | |
|
Median OS in months [95% CI] |
29.80 [22.87, 45.67] |
17.41 [13.83, 27.70] |
Figure 3: ELOQUENT-3 Progression-Free Survival
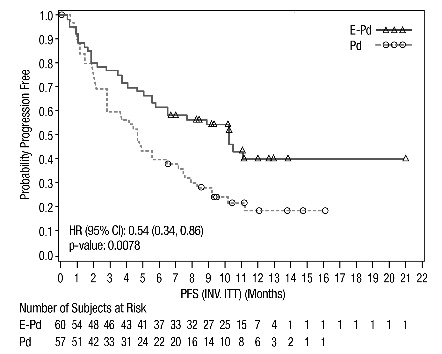
Figure 4: ELOQUENT-3 Overall Survival
****
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
EMPLICITI (elotuzumab) is white to off-white lyophilized powder available as follows:
|
Carton Content |
NDC |
|
One 300 mg single-dose vial |
0003-2291-11 |
|
One 400 mg single-dose vial |
0003-4522-11 |
Store EMPLICITI under refrigeration at 2°C to 8°C (36°F-46°F). Protect EMPLICITI from light by storing in the original package until time of use. Do not freeze or shake.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Infusion Reactions
•
EMPLICITI may cause infusion reactions. Advise patients to contact their healthcare provider if they experience signs and symptoms of infusion reactions, including fever, chills, rash, or breathing problems within 24 hours of infusion [see Warnings and Precautions (5.1)].
•
Advise patients that they will be required to take the following oral medications prior to EMPLICITI dosing to reduce the risk of infusion reaction [see Dosage and Administration (2.3)]:
•
Dexamethasone orally as prescribed
•
H1 blocker: diphenhydramine or equivalent (if oral)
•
H2 blocker: ranitidine or equivalent (if oral)
•
Acetaminophen (650 to 1000 mg orally)
Pregnancy
•
Advise patients that lenalidomide and pomalidomide have the potential to cause fetal harm and has specific requirements regarding contraception, pregnancy testing, blood and sperm donation, and transmission in sperm. Lenalidomide and pomalidomide are only available through a REMS program [see Use in Specific Populations (8.1)].
Infections
•
Inform patients of the risk of developing infections during treatment with EMPLICITI, and to report any symptoms of infection [see Warnings and Precautions (5.2)].
Second Primary Malignancies
•
Inform patients of the risk of developing SPM during treatment with EMPLICITI [see Warnings and Precautions (5.3)].
Hepatotoxicity
•
Inform patients of the risk of hepatotoxicity during treatment with EMPLICITI and to report any signs and symptoms associated with this event to their healthcare provider for evaluation [see Warnings and Precautions (5.4)].
Manufactured by:
Bristol-Myers Squibb Company
Princeton, NJ 08543 USA
U.S. License No. 1713
SPL PATIENT PACKAGE INSERT SECTION
|
Patient Information EMPLICITI**®** (em-plis-city) (elotuzumab) for injection | |
|
EMPLICITI is used with other prescription medicines called REVLIMID**®** (lenalidomide) and dexamethasone or POMALYST**®** (pomalidomide) and dexamethasone.**Read the Medication Guide that comes with REVLIMID if used with REVLIMID and POMALYST if used with POMALYST.**You can ask your healthcare provider or pharmacist for information about dexamethasone. | |
|
What is EMPLICITI? EMPLICITI is a prescription medicine used to treat: • • It is not known if EMPLICITI is safe and effective in children. | |
|
Before you receive EMPLICITI, tell your healthcare provider about all of your medical conditions, including if you: • • o o • • | |
|
How will I receive EMPLICITI? • • • o o • o o • • • | |
|
What are the possible side effects of EMPLICITI? EMPLICITI may cause serious side effects, including: • | |
|
o o o o |
o o o |
|
• | |
|
o o o |
o o o |
|
• | |
|
• The most common side effects of EMPLICITI when used with REVLIMID and dexamethasone include: | |
|
• • • • • |
• • • • • |
|
The most common side effects of EMPLICITI when used with POMALYST and dexamethasone include: • • These are not all of the possible side effects of EMPLICITI. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. You may also report side effects to Bristol-Myers Squibb at 1-800-721-5072. | |
|
General information about the safe and effective use of EMPLICITI Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. You can ask your pharmacist or healthcare provider for information about EMPLICITI that is written for health professionals. | |
|
What are the ingredients of EMPLICITI? **Active ingredient:**elotuzumab Inactive ingredients: citric acid monohydrate, polysorbate 80, sodium citrate, sucrose For more information, call 1-844-EMPLICITI (844-367-5424) or visit EMPLICITI.com. EMPLICITI® is a trademark of Bristol-Myers Squibb Company. All other trademarks are the property of their respective owners. Manufactured by: Bristol-Myers Squibb Company, Princeton, NJ 08543 USA U.S. License No. 1713 |
This Patient Information has been approved by the U.S. Food and Drug Administration.
Revised: 3/2022
