ARZERRA
These highlights do not include all the information needed to use ARZERRA safely and effectively. See full prescribing information for ARZERRA. ARZERRA (ofatumumab) injection, for intravenous useInitial U.S. Approval: 2009
77785ce3-e8df-4ca1-8f8e-6c418c6a17de
HUMAN PRESCRIPTION DRUG LABEL
Aug 2, 2023
Novartis Pharmaceuticals Corporation
DUNS: 002147023
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
ofatumumab
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
ofatumumab
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
Drug Labeling Information
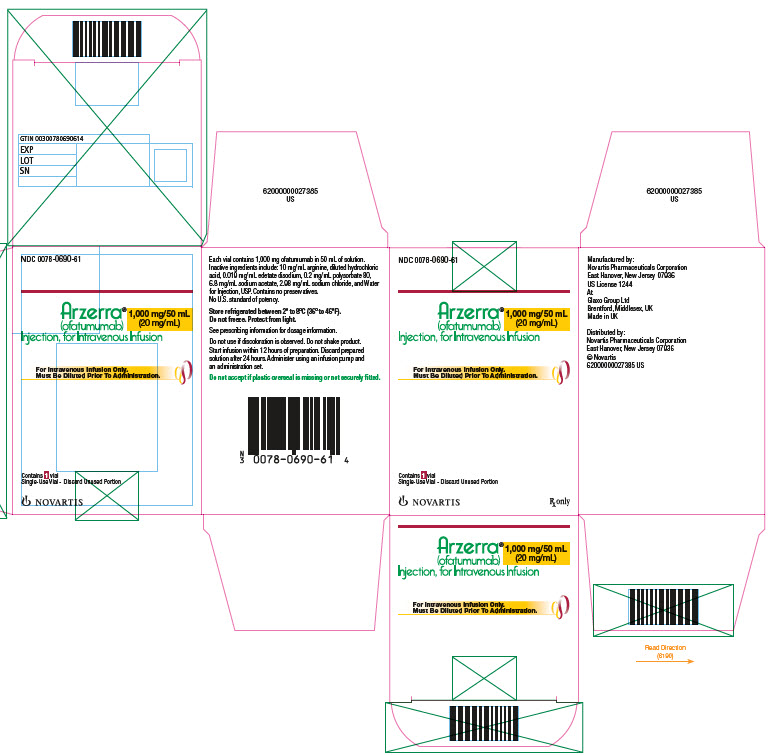
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Principal Display Panel
NDC 0078-0690-61
Arzerra**®**
(ofatumumab)
Injection, for Intravenous Infusion
1000 mg/50 mL(20mg/mL)
Rx only
For Intravenous Infusion Only.
Must Be Diluted Prior To Administration.
Contains1 vial
Single-Use Vials - Discard Unused Portion
Novartis
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
ARZERRA may cause fetal B-cell depletion based on findings from animal studies and the drug’s mechanism of action [see Clinical Pharmacology (12.1)]. There are no data on ARZERRA use in pregnant women to inform a drug-associated risk. However, there are clinical considerations [see Clinical Considerations]. No teratogenicity was observed in animal reproduction studies with administration of ARZERRA to pregnant monkeys during organogenesis at doses 0.3 and 2.4 times the maximum recommended human dose (MRHD) of 2000 mg based on monkey geometric mean AUCinf of 213 mg.h/mL and 1646 mg.h/mL, respectively. However, ofatumumab caused depletion of maternal circulating B-cells, depletion of peripheral and splenic fetal B-cells, and decreased fetal spleen weights [see Data].
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population the estimated background risk of major birth defects is 2% to 4% and of miscarriage is 15% to 20% of clinically recognized pregnancies, respectively.
Clinical Considerations
Fetal/neonatal adverse reactions
ARZERRA may cause fetal B-cell depletion [see Data]. Avoid administering live vaccines to neonates and infants exposed to ARZERRA in utero until B-cell recovery occurs [see Warnings and Precautions (5.7) and Clinical Pharmacology (12.2)].
Data
Animal Data
In an embryo-fetal development study, pregnant cynomolgus monkeys received 20 or 100 mg/kg/day of ofatumumab intravenously (30 minute infusion) once weekly during the period of organogenesis [Gestation Days (GD) 20 to 50] with systemic exposure throughout pregnancy due to the long half-life as drug was detected in maternal serum on GD 100 (early fetal period of development). At the end of organogenesis on GD 48, the exposure in pregnant monkeys receiving ofatumumab 20 and 100 mg/kg/day was approximately 0.3 and 2.4 times the human exposure after the 8th infusion of the MRHD of 2,000 mg based on monkey geometric mean AUCinf of 213 mg.h/mL and 1,646 mg.h/mL, respectively. Ofatumumab crossed the placenta, as it was detected in fetal cord blood on GD 100 in both dose groups. There was no maternal toxicity and no effect on pregnancy success. As expected, both dose levels of ofatumumab depleted circulating B cells in the mothers; recovery of B lymphocytes in dosed animals was not observed during the dosing-free period. Following Caesarean section at GD 100, fetuses from treated mothers exhibited decreases in mean peripheral and splenic B-cell counts (decreased to approximately 12% and 15% of control values at both dose levels, respectively and spleen weights (decreased by approximately 15% in low-dose group and by approximately 30% in high-dose group, compared with control values). There was no effect on prenatal survival and no evidence of teratogenicity in this monkey study.
The kinetics of B-lymphocyte recovery and the potential long-term effects of perinatal B-cell depletion in offspring from ofatumumab-treated maternal monkeys have not been studied.
8.2 Lactation
Risk Summary
There is no information regarding the presence of ARZERRA in human milk, the effects on the breastfed infant, or the effects on milk production. Human IgG is known to be present in human milk. Published data suggest that antibodies in breast milk do not enter the neonatal and infant circulations in substantial amounts.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for ARZERRA and any potential adverse effects on the breastfed infant from ARZERRA or from the underlying maternal condition.
8.4 Pediatric Use
Safety and effectiveness of ARZERRA have not been established in children.
8.5 Geriatric Use
In patients with previously untreated CLL (Study 1), 68% (148/217) receiving ARZERRA plus chlorambucil were 65 years and older. Patients age 65 years and older experienced a higher incidence of the following Grade 3 or greater adverse reactions compared with patients younger than 65 years of age: neutropenia (30% versus 17%) and pneumonia (5% versus 1%) [see Adverse Reactions (6.1)]. In patients 65 years and older, 29% experienced serious adverse events compared with 13% of patients younger than 65 years. No clinically meaningful differences in the effectiveness of ARZERRA plus chlorambucil were observed between older and younger patients [see Clinical Studies (14.1)].
In patients with relapsed CLL (Study 2), 33% (60/181) of patients receiving ARZERRA plus fludarabine and cyclophosphamide were 65 years and older. No clinically meaningful differences in the safety or effectiveness of ARZERRA were observed between patients age 65 years and older, and those younger than 65 years [see Adverse Reactions (6.1), Clinical Studies (14.2)].
With extended treatment in patients with CLL (Study 3), 49% (117/237) receiving ARZERRA were 65 years and older. No clinically meaningful differences in the safety or effectiveness of ARZERRA were observed between patients age 65 years and older and those younger than 65 years of age [see Adverse Reactions (6.1), Clinical Studies (14.3)].
In refractory CLL clinical studies there were insufficient numbers of patients aged 65 years and older to determine whether they respond differently from younger patients [see Clinical Pharmacology (12.3)].
- Pregnancy: May cause fetal B-cell depletion. (8.1)
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Ofatumumab binds specifically to both the small and large extracellular loops of the CD20 molecule. The CD20 molecule is expressed on normal B lymphocytes (pre–B- to mature B-lymphocyte) and on B-cell CLL. The CD20 molecule is not shed from the cell surface and is not internalized following antibody binding.
The Fab domain of ofatumumab binds to the CD20 molecule and the Fc domain mediates immune effector functions to result in B-cell lysis in vitro. Data suggest that possible mechanisms of cell lysis include complement-dependent cytotoxicity and antibody-dependent, cell-mediated cytotoxicity.
12.2 Pharmacodynamics
B-cell Depletion: In patients with previously untreated CLL, at 6 months after the last dose, the median reductions in CD19-positive B cells were >99% (n = 155) for ARZERRA in combination with chlorambucil and 94% (n = 121) for chlorambucil alone.
In patients with relapsed CLL, the proportion of responders in the ofatumumab in combination with fludarabine and cyclophosphamide (O+FC) arm who showed complete or near complete B cell depletion was 39% (n = 59) and 82% (n = 126) , respectively. The proportion of responders in the fludarabine and cyclophosphamide (FC) arm with complete or near complete B cell depletion was 4% (n = 5) and 23% (n = 28), respectively.
In patients treated with extended treatment for CLL after response to therapy for their recurrent or progressive disease, the median decreases in B-cell counts were 61% (n = 168) after the first infusion and 80% (n = 114) prior to the sixth infusion; in the observation arm, the median changes in B-cell counts at the same time points were increases of 32% (n = 148) and 1,328% (n = 95).
In patients with CLL refractory to fludarabine and alemtuzumab, the median decrease in circulating CD19-positive B cells was 91% (n = 50) with the 8th infusion and 85% (n = 32) with the 12th infusion. The time to recovery of lymphocytes, including CD19-positive B cells, to normal levels has not been determined.
Although the depletion of B-cells in the peripheral blood is a measurable pharmacodynamic effect, it is not directly correlated with the depletion of B cells in solid organs or in malignant deposits. B-cell depletion has not been shown to be directly correlated to clinical response.
Cardiac Electrophysiology: The effect of multiple doses of ARZERRA on the QTc interval was evaluated in a pooled analysis of 3 open-label studies in patients with CLL (N = 85). Patients received ARZERRA 300 mg on Day 1 followed by either 1,000 mg or 2,000 mg for subsequent doses. No large changes in the mean QTc interval (i.e., >20 milliseconds) were detected in the pooled analysis.
12.3 Pharmacokinetics
Ofatumumab is eliminated through both a target-independent route and a B cell- mediated route. Ofatumumab exhibited dose-dependent clearance in the dose range of 100 to 2,000 mg. Due to the depletion of B cells, the clearance of ofatumumab decreased substantially after subsequent infusions compared with the first infusion.
Pharmacokinetic data were obtained after repeated administration (4, 5, 6, 8, or 12 infusions) of 1,000 mg or 2,000 mg doses in 774 patients with CLL (Studies 1, 2, 3, 4 and 5). The geometric mean (% CV) values for clearance, volume of distribution at steady state (Vss), and half-life for ofatumumab in these patients were 9.3 mL/hour (91%), 6.1 L (52%), and 17.6 days (83%). The pharmacokinetic profile was similar across doses in patients with CLL.
Specific Populations: The following population characteristics do not have a clinically meaningful effect on the pharmacokinetics of ofatumumab: body size, gender, age, and renal impairment (evaluated in patients with a calculated creatinine clearance ≥30 mL/min).
No formal studies of ARZERRA in patients with hepatic impairment have been conducted. The effect of a calculated CrCL < 30 mL/min on the pharmacokinetics of ARZERRA has not been evaluated.
Drug Interactions: Coadministration of ARZERRA did not result in clinically relevant effects on the pharmacokinetics of fludarabine, cyclophosphamide, chlorambucil, or its active metabolite, phenylacetic acid mustard.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No carcinogenicity or mutagenicity studies of ofatumumab have been conducted. In a repeat-dose toxicity study, no tumorigenic or unexpected mitogenic responses were noted in cynomolgus monkeys treated for 7 months with up to 3.5 times the maximum human dose (2,000 mg) of ofatumumab. Effects on male and female fertility have not been evaluated in animal studies.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
ARZERRA (ofatumumab) is a sterile, clear to opalescent, colorless, preservative-free liquid concentrate (20 mg/mL) for dilution and intravenous administration provided in single-use glass vials with a rubber stopper (not made with natural rubber latex) and an aluminum overseal. Each vial contains either 100 mg ofatumumab in 5 mL of solution or 1,000 mg ofatumumab in 50 mL of solution.
ARZERRA is available as follows:
|
Carton Contents |
NDC |
|
3 single-use 100 mg/5 mL vials |
Vial: NDC 0078-0669-61 Carton of 3 vials: NDC 0078-0669-13 |
|
1 single-use 1,000 mg/50 mL vial |
Vial and Carton: NDC 0078-0690-61 |
Store ARZERRA refrigerated between 2° to 8°C (36° to 46°F). Do not freeze. Vials should be protected from light.
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage Regimen
- Dilute and administer as an intravenous infusion according to the following schedules.
- Do not administer as an intravenous push or bolus or as a subcutaneous injection.
- Pre-medicate before each infusion [see Dosage and Administration (2.4)].
Previously Untreated CLL:
The recommended dosage and schedule in combination with chlorambucil is:
- 300 mg on Day 1, followed 1 week later by 1,000 mg on Day 8 (Cycle 1), followed by
- 1,000 mg on Day 1 of subsequent 28-day cycles for a minimum of 3 cycles until best response or a maximum of 12 cycles.
Relapsed CLL:
The recommended dosage and schedule in combination with fludarabine and cyclophosphamide is:
- 300 mg on Day 1, followed 1 week later by 1,000 mg on Day 8 (Cycle 1), followed by
- 1,000 mg on Day 1 of subsequent 28-day cycles for a maximum of 6 cycles.
Extended Treatment in CLL: The recommended dosage and schedule as single-agent extended treatment in CLL is:
- 300 mg on Day 1, followed by
- 1,000 mg 1 week later on Day 8, followed by
- 1,000 mg 7 weeks later and every 8 weeks thereafter for up to a maximum of 2 years.
Refractory CLL: The recommended dosage and schedule is 12 doses administered as follows:
- 300 mg initial dose on Day 1, followed 1 week later by
- 2,000 mg weekly for 7 doses (Infusions 2 through 8), followed 4 weeks later by
- 2,000 mg every 4 weeks for 4 doses (Infusions 9 through 12).
2.2 Administration
Administer ARZERRA in an environment where facilities to adequately monitor and treat infusion reactions are available [see Warnings and Precautions (5.1)].
Prepare all doses in 1,000 mL of 0.9% Sodium Chloride Injection, USP [see Dosage and Administration (2.5)].
Previously Untreated CLL, Relapsed CLL, and Extended Treatment in CLL:
- For initial 300-mg dose: Initiate infusion at a rate of 3.6 mg/hour (12 mL/hour).
- For subsequent infusions of 1,000 mg: Initiate infusion at a rate of 25 mg/hour (25 mL/hour). Initiate infusion at a rate of 12 mg/hour if a Grade 3 or greater infusion-related adverse event was experienced during the previous infusion.
In the absence of an infusion-related adverse event, the rate of infusion may be increased every 30 minutes (Table 1). Do not exceed the infusion rates in Table 1.
Table 1. Infusion Rates for ARZERRA in Previously Untreated CLL, Relapsed CLL, and Extended Treatment in CLL|
a Initial 300 mg: median durations of infusions = 4.8 to 5.2 hours. | ||
|
Interval After Start of Infusion (min) |
Initial 300 mg Dose****a (mL/hour) |
Subsequent Infusions****b (mL/hour) |
|
0-30 |
12 |
25 |
|
31-60 |
25 |
50 |
|
61-90 |
50 |
100 |
|
91-120 |
100 |
200 |
|
121-150 |
200 |
400 |
|
151-180 |
300 |
400 |
|
400 |
400 |
Refractory CLL:
- Infusion 1 (300-mg dose): Initiate infusion at a rate of 3.6 mg/hour (12 mL/hour).
- Infusion 2 (2,000-mg dose): Initiate infusion at a rate of 24 mg/hour (12 mL/hour).
- Infusions 3 through 12 (2,000-mg doses): Initiate infusion at a rate of 50 mg/hour (25 mL/hour).
In the absence of an infusion-related adverse event, the rate of infusion may be increased every 30 minutes (Table 2). Do not exceed the infusion rates in Table 2.
Table 2. Infusion Rates for ARZERRA in Refractory CLL|
a Infusions 1 and 2 (300 mg and 2,000 mg): median duration of infusions = 6.8
hours. | ||
|
Interval after Start of Infusion (min) |
Infusions 1 and 2****a (mL/hour) |
Subsequent Infusions****b (mL/hour) |
|
0-30 |
12 |
25 |
|
31-60 |
25 |
50 |
|
61-90 |
50 |
100 |
|
91-120 |
100 |
200 |
|
200 |
400 |
2.3 Infusion Rate Dose Modification for Infusion Reactions
- Interrupt infusion for infusion reactions of any severity [see Warnings and Precautions (5.1)]. Treatment can be resumed at the discretion of the treating physician. The following infusion rate modifications can be used as a guide.
- If the infusion reaction resolves or remains less than or equal to Grade 2, resume infusion with the following modifications according to the initial Grade of the infusion reaction.
- Grade 1 or 2: Infuse at one‑half of the previous infusion rate.
- Grade 3 or 4: Infuse at a rate of 12 mL/hour.
- After resuming the infusion, the infusion rate may be increased according to Tables 1 and 2 above, based on patient tolerance.
- Consider permanent discontinuation of ARZERRA if the severity of the infusion reaction does not resolve to less than or equal to Grade 2 despite adequate clinical intervention.
- Permanently discontinue therapy for patients who develop an anaphylactic reaction to ARZERRA.
2.4 Premedication
Patients should receive all of the following premedication agents 30 minutes to 2 hours prior to each infusion of ARZERRA. See Table 3 for pre-medication schedule prior to each infusion.
Table 3. Premedication Schedule for ARZERRA|
a Up to 13 infusions in previously untreated CLL; up to 7 infusions in
relapsed CLL, up to 14 infusions in extended treatment in CLL. | |||||
|
Previously Untreated CLL, Relapsed CLL or Extended Treatment in CLL |
Refractory CLL | ||||
|
Infusion Number |
1 and 2 |
3 and beyond****a |
1, 2, and 9 |
3 to 8 |
10 to 12 |
|
Intravenous corticosteroid (prednisolone or equivalent) |
50 mg |
0-50 mgb |
100 mg |
0-100 mgb |
50-100 mgc |
|
Oral acetaminophen |
1,000 mg | ||||
|
Oral or intravenous antihistamine |
Diphenhydramine 50 mg or cetirizine 10 mg (or equivalent) |
2.5 Preparation and Administration
- Do not shake product.
- Inspect parenteral drug products visually for particulate matter and discoloration prior to administration. ARZERRA should be a clear to opalescent, colorless solution. The solution should not be used if discolored or cloudy, or if foreign particulate matter is present.
Preparation of Solution:
- 300-mg dose: Withdraw and discard 15 mL from a 1,000-mL bag of 0.9% Sodium Chloride Injection, USP. Withdraw 5 mL from each of 3 single-use 100-mg vials of ARZERRA and add to the bag. Mix diluted solution by gentle inversion.
- 1,000-mg dose: Withdraw and discard 50 mL from a 1,000-mL bag of 0.9% Sodium Chloride Injection, USP. Withdraw 50 mL from 1 single-use 1,000-mg vial of ARZERRA and add to the bag. Mix diluted solution by gentle inversion.
- 2,000-mg dose: Withdraw and discard 100 mL from a 1,000-mL bag of 0.9% Sodium Chloride Injection, USP. Withdraw 50 mL from each of 2 single-use 1,000-mg vials of ARZERRA and add to the bag. Mix diluted solution by gentle inversion.
- Store diluted solution between 2° to 8°C (36° to 46°F).
- No incompatibilities between ARZERRA and polyvinylchloride or polyolefin bags and administration sets have been observed.
Administration Instructions:
- Do not mix ARZERRA with, or administer as an infusion with other medicinal products.
- Administer using an infusion pump and an administration set.
- Flush the intravenous line with 0.9% Sodium Chloride Injection, USP before and after each dose.
- Start infusion within 12 hours of preparation.
- Discard prepared solution after 24 hours.
- Dilute and administer as an intravenous infusion. Do not administer subcutaneously or as an intravenous push or bolus. (2.1)
- Previously untreated CLL in combination with chlorambucil recommended dosage and schedule is:
- 300 mg on Day 1 followed by 1,000 mg on Day 8 (Cycle 1)
- 1,000 mg on Day 1 of subsequent 28-day cycles for a minimum of 3 cycles until best response or a maximum of 12 cycles. (2.1)
- Relapsed CLL in combination with fludarabine and cyclophosphamide recommended dosage and schedule is:
- 300 mg on Day 1 followed by 1,000 mg on Day 8 (Cycle 1)
- 1,000 mg on Day 1 of subsequent 28-day cycles for a maximum of 6 cycles (2.1)
- Extended treatment in CLL recommended dosage and schedule is:
- 300 mg on Day 1 followed by
- 1,000 mg 1 week later on Day 8, followed by
- 1,000 mg 7 weeks later and every 8 weeks thereafter for up to a maximum of 2 years. (2.1)
- Refractory CLL recommended dosage and schedule is:
- 300 mg initial dose, followed 1 week later by
- 2,000 mg weekly for 7 doses, followed 4 weeks later by
- 2,000 mg every 4 weeks for 4 doses. (2.1)
- Administer where facilities to adequately monitor and treat infusion reactions are available. (2.2)
- Pre-medicate with acetaminophen, antihistamine, and corticosteroid. (2.4)
