Polyethylene Glycol 3350, Sodium Chloride, Sodium Bicarbonate and Potassium Chloride - Unflavored
These highlights do not include all the information needed to use POLYETHYLENE GLYCOL 3350, SODIUM CHLORIDE, SODIUM BICARBONATE and POTASSIUM CHLORIDE for Oral Solution safely and effectively. See full prescribing information for POLYETHYLENE GLYCOL 3350, SODIUM CHLORIDE, SODIUM BICARBONATE and POTASSIUM CHLORIDE for Oral Solution. POLYETHYLENE GLYCOL 3350, SODIUM CHLORIDE, SODIUM BICARBONATE and POTASSIUM CHLORIDE for Oral Solution (UNFLAVORED, LEMON AND ORANGE FLAVORS) Initial U.S. Approval: 1991
37c111ec-5b62-42b9-bbb2-3b0c530e34c9
HUMAN PRESCRIPTION DRUG LABEL
Nov 9, 2022
Strides Pharma Science Limited
DUNS: 650738743
Products 3
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Polyethylene Glycol 3350, Sodium Chloride, Sodium Bicarbonate and Potassium Chloride with Orange Flavor
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Polyethylene Glycol 3350, Sodium Chloride, Sodium Bicarbonate and Potassium Chloride - Unflavored
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (4)
Polyethylene Glycol 3350, Sodium Chloride, Sodium Bicarbonate and Potassium Chloride with Lemon Flavor
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Drug Labeling Information
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Serious Fluid and Serum Chemistry Abnormalities
Advise patients to hydrate adequately before, during, and after the use of polyethylene glycol 3350, sodium chloride, sodium bicarbonate and potassium chloride for oral solution. Use caution in patients with congestive heart failure when replacing fluids. If a patient develops significant vomiting or signs of dehydration including signs of orthostatic hypotension after taking polyethylene glycol 3350, sodium chloride, sodium bicarbonate and potassium chloride for oral solution, consider performing post-colonoscopy lab tests (electrolytes, creatinine, and BUN) and treat accordingly. Fluid and electrolyte disturbances can lead to serious adverse events including cardiac arrhythmias, seizures and renal impairment. Fluid and electrolyte abnormalities should be corrected before treatment with polyethylene glycol 3350, sodium chloride, sodium bicarbonate and potassium chloride for oral solution.
In addition, use caution when prescribing polyethylene glycol 3350, sodium chloride, sodium bicarbonate and potassium chloride for oral solution for patients who have conditions, or who are using medications, that increase the risk for fluid and electrolyte disturbances or may increase the risk of adverse events of seizure, arrhythmias, and renal impairment [see Drug Interactions (7.1)].
5.2 Cardiac Arrhythmias
There have been rare reports of serious arrhythmias associated with the use of ionic osmotic laxative products for bowel preparation. Use caution when prescribing polyethylene glycol 3350, sodium chloride, sodium bicarbonate and potassium chloride for oral solution for patients at increased risk of arrhythmias (e.g., patients with a history of prolonged QT, uncontrolled arrhythmias, recent myocardial infarction, unstable angina, congestive heart failure, or cardiomyopathy). Pre-dose and post-colonoscopy ECGs should be considered in patients at increased risk of serious cardiac arrhythmias.
5.3 Seizures
There have been reports of generalized tonic-clonic seizures and/or loss of consciousness associated with use of bowel preparation products in patients with no prior history of seizures. The seizure cases were associated with electrolyte abnormalities (e.g., hyponatremia, hypokalemia, hypocalcemia, and hypomagnesemia) and low serum osmolality. The neurologic abnormalities resolved with correction of fluid and electrolyte abnormalities.
Use caution when prescribing polyethylene glycol 3350, sodium chloride, sodium bicarbonate and potassium chloride for oral solution for patients with a history of seizures and in patients at increased risk of seizure, such as patients taking medications that lower the seizure threshold (e.g., tricyclic antidepressants), patients withdrawing from alcohol or benzodiazepines, or patients with known or suspected hyponatremia.
5.4 Renal Impairment
Use caution when prescribing polyethylene glycol 3350, sodium chloride, sodium bicarbonate and potassium chloride for oral solution for patients with impaired renal function or patients taking concomitant medications that may affect renal function (such as diuretics, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, or non-steroidal anti-inflammatory drugs). Advise these patients of the importance of adequate hydration, and consider performing baseline and post-colonoscopy laboratory tests (electrolytes, creatinine, and BUN) in these patients.
5.5 Colonic Mucosal Ulcerations and Ischemic Colitis
Administration of osmotic laxative products may produce colonic mucosal aphthous ulcerations, and there have been reports of more serious cases of ischemic colitis requiring hospitalization. Concurrent use of stimulant laxatives and polyethylene glycol 3350, sodium chloride, sodium bicarbonate and potassium chloride for oral solution may increase this risk. The potential for mucosal ulcerations resulting from the bowel preparation should be considered when interpreting colonoscopy findings in patients with known or suspect inflammatory bowel disease (IBD).
5.6 Use in Patients with Significant Gastrointestinal Disease
If gastrointestinal obstruction or perforation is suspected, perform appropriate diagnostic studies to rule out these conditions before administering polyethylene glycol 3350, sodium chloride, sodium bicarbonate and potassium chloride for oral solution. If a patient experiences severe bloating, distention or abdominal pain, administration should be slowed or temporarily discontinued until the symptoms abate. If gastrointestinal obstruction or perforation is suspected, appropriate studies should be performed to rule out these conditions before administration of polyethylene glycol 3350, sodium chloride, sodium bicarbonate and potassium chloride for oral solution.
Use with caution in patients with severe active ulcerative colitis.
5.7 Aspiration
Use with caution in patients with impaired gag reflex, unconscious, or semiconscious patients, and patients prone to regurgitation or aspiration. Such patients should be observed during administration of polyethylene glycol 3350, sodium chloride, sodium bicarbonate and potassium chloride for oral solution, especially if it is administered via nasogastric tube.
Do not combine polyethylene glycol 3350, sodium chloride, sodium bicarbonate and potassium chloride for oral solution, with starch-based thickeners [see Dosage and Administration (2.1)]. Polyethylene glycol (PEG), a component of polyethylene glycol 3350, sodium chloride, sodium bicarbonate and potassium chloride for oral solution, when mixed with starch-thickened liquids reduces the viscosity of the starch-thickened liquid. When a PEG-based product used for another indication was mixed in starch-based pre-thickened liquids used in patients with dysphagia, thinning of the liquid occurred and cases of choking and potential aspiration were reported.
5.8 Not for Direct Ingestion
The contents of each jug must be diluted with water to a final volume of 4 liters (4 L) and ingestion of additional water is important to patient tolerance. Direct ingestion of the undissolved powder may increase the risk of nausea, vomiting, dehydration, and electrolyte disturbances.
- Risk of fluid and electrolyte abnormalities, arrhythmias, seizures and renal impairment – assess concurrent medications and consider testing in some patients (5.1, 5.2, 5.3, 5.4)
- Patients with renal insufficiency – use caution, ensure adequate hydration and consider testing (5.4)
- Suspected GI obstruction or perforation – rule out the diagnosis before administration (4, 5.6)
- Patients at risk for aspiration – observe during administration (5.7)
- Not for direct ingestion – dilute and take with additional water (5.8)
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Drugs that May Lead to Fluid and Electrolyte Abnormalities
Use caution when prescribing polyethylene glycol 3350, sodium chloride, sodium bicarbonate and potassium chloride for oral solution for patients who are using medications that increase the risk for fluid and electrolyte disturbances or may increase the risk of adverse events of seizure, arrhythmias, and prolonged QT in the setting of fluid and electrolyte abnormalities. Consider additional patient evaluations as appropriate [see Warnings and Precautions (5.1, 5.2, 5.3, and 5.4)] in patients taking these concomitant medications.
7.2 Potential for Altered Drug Absorption
Oral medication administered within one hour of the start of administration of polyethylene glycol 3350, sodium chloride, sodium bicarbonate and potassium chloride for oral solution may be flushed from the gastrointestinal tract and the medication may not be absorbed properly.
7.3 Stimulant Laxatives
Concurrent use of stimulant laxatives and polyethylene glycol 3350, sodium chloride, sodium bicarbonate and potassium chloride for oral solution may increase the risk of mucosal ulceration or ischemic colitis. Avoid use of stimulant laxatives (e.g., bisacodyl, sodium picosulfate) while taking polyethylene glycol 3350, sodium chloride, sodium bicarbonate and potassium chloride for oral solution.
- Some drugs increase risks due to fluid and electrolyte changes (7.1)
- Oral medication taken within 1 hour of start of each dose may not be absorbed properly (7.2)
DESCRIPTION SECTION
11 DESCRIPTION
For oral solution: Each 5 liter (5L) polyethylene glycol 3350, sodium chloride, sodium bicarbonate and potassium chloride for oral solution jug contains a white powder for reconstitution. Polyethylene glycol 3350, sodium chloride, sodium bicarbonate and potassium chloride for oral solution is a combination of polyethylene glycol 3350, an osmotic laxative, and electrolytes (sodium chloride, sodium bicarbonate and potassium chloride) for oral solution. Polyethylene glycol 3350, sodium chloride, sodium bicarbonate and potassium chloride for oral solution is available in unflavored, lemon and orange flavors.
Each 5 liter jug contains: polyethylene glycol 3350 USP-NF 420 g, sodium bicarbonate USP 5.72 g, sodium chloride USP 11.2 g, potassium chloride USP 1.48 g. Besides these, the lemon flavored powder contains flavoring ingredients acesulfame potassium 0.1 gram and flavor lemon 0.4 grams while orange flavor powder contains acesulfame potassium 0.1 gram and flavor orange 0.6 grams respectively. The solution is clear and colorless when reconstituted to a final volume of 4 liters with water.
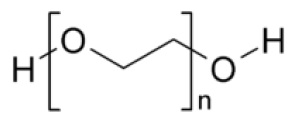
Polyethylene Glycol 3350, NF
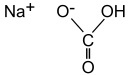
Sodium Bicarbonate, USP
The chemical name is NaHCO3. The average Molecular Weight is 84.01. The structural formula is:
Sodium Chloride, USP
The chemical name is NaCl. The average Molecular Weight: 58.44. The structural formula is:
Na+ Cl-
Potassium Chloride, USP
The chemical name is KCl. The average Molecular Weight: 74.55. The structural formula is:
K-Cl
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
In powdered form, for oral administration as a solution following reconstitution.
Polyethylene glycol 3350, sodium chloride, sodium bicarbonate and potassium chloride for oral solution is available in a disposable jug in powdered form containing:
Polyethylene glycol****3350, sodium chloride, sodium bicarbonate and potassium chloride for oral solution with Flavor Packs: polyethylene glycol 3350 420 g, sodium bicarbonate 5.72 g, sodium chloride 11.2 g, potassium chloride 1.48 g and flavoring ingredients acesulfame potassium 0.1 gram and flavor lemon 0.4 grams for lemon flavor and acesulfame potassium 0.1 gram and flavor orange 0.6 grams for orange flavor. When made up to 4 liters volume with water, the solution contains PEG-3350 31.3 mmol/L, sodium 65 mmol/L, chloride 53 mmol/L, bicarbonate 17 mmol/L and potassium 5 mmol/L.
Storage:
Store at 25°C (77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]. When reconstituted, keep solution refrigerated. Use within 48 hours. Discard unused portion.
Keep out of reach of children.
Polyethylene glycol 3350, sodium chloride, sodium bicarbonate and potassium chloride for oral solution is available in following flavors:
|
** Drug product** |
** NDC** |
** Package** |
|
Polyethylene glycol 3350, sodium chloride, sodium bicarbonate and potassium chloride for oral solution |
64380-768-21 | |
|
Polyethylene glycol 3350, sodium chloride, sodium bicarbonate and potassium chloride for oral solution with lemon flavor |
64380-769-21 |
5 L disposable jug with a 4 L fill line |
|
Polyethylene glycol 3350, sodium chloride, sodium bicarbonate and potassium chloride for oral solution with orange flavor |
64380-770-21 |
