Ursodiol
Ursodiol Capsules, USP Rx only Prescribing Information SPECIAL NOTE Gallbladder stone dissolution with ursodiol treatment requires months of therapy. Complete dissolution does not occur in all patients and recurrence of stones within 5 years has been observed in up to 50% of patients who do dissolve their stones on bile acid therapy. Patients should be carefully selected for therapy with ursodiol, and alternative therapies should be considered.
631339fc-548d-4cd5-8cf3-60e1590f79f6
HUMAN PRESCRIPTION DRUG LABEL
Sep 7, 2023
Aurobindo Pharma Limited
DUNS: 650082092
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Ursodiol
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 300 mg (100 Capsules Bottle)
NDC 59651-421-01
** Rx only**
** Ursodiol Capsules, USP**
** 300 mg**
AUROBINDO 100 Capsules
****
DESCRIPTION SECTION
DESCRIPTION
Ursodiol capsules, USP are a bile acid available as 300 mg capsules suitable for oral administration.
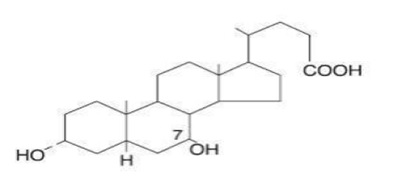
Ursodiol USP (ursodeoxycholic acid), a naturally occurring bile acid found in small quantities in normal human bile and in the biles of certain other mammals. It is a bitter-tasting, white or almost white crystalline powder practically insoluble in water; freely soluble in ethanol (96 percent), slightly soluble in acetone, practically insoluble in methylene chloride. The chemical name for ursodiol is 3α,7β-dihydroxy-5β-cholan-24-oic acid (C24H40O4). Ursodiol USP has a molecular weight of 392.58. Its structure is shown below:
Inactive Ingredients: Colloidal silicon dioxide, corn starch and magnesium stearate. The hard gelatin capsule shells contain gelatin, iron oxide red and titanium dioxide. The capsules are imprinted with black ink containing black iron oxide, potassium hydroxide and shellac.
