MTL1 Leucoflex
Anticoagulant Citrate Phosphate Dextrose Solution USP (CPD) with an integral container of Additive Solution (AS-1) preservative, and an integral Leucoflex MTL1 Leukocyte Reduction Filter for Whole Blood
0a0b25d1-8fa1-6cd4-e054-00144ff8d46c
HUMAN PRESCRIPTION DRUG LABEL
Nov 10, 2023
Maco Productions
DUNS: 265492868
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Anticoagulant Citrate Phosphate Dextrose Solution with AS-1
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Labels:
** Bag 1 Anticoagulant Citrate Phosphate Dextrose Solution USP; Bag 4 Additive Solution AS-1; Overwrap; Carton**
Anticoagulant Citrate Phosphate Dextrose Solution, USP - Bag 1 Label

Additive Solution AS-1 - Bag 4 Label
OVERWRAP LABEL

CARTON LABEL

DESCRIPTION SECTION
Description
The Leucoflex MTL1 product consists of a blood collection system with 70mL of CPD for the collecion of 500 +/- 50mL of blood and 110mL of AS-1 Additive Solution. They are supplied with sterile, non-pyrogenic fluid pathways. The product as supplied includes an in-line leukoreduction filter known as Leucoflex MTL1, storage containers for blood components and tubing, a sample diversion pouch (Bactivam), a vacuum tube adapter (Vacuvam), and a protective shield for the used needle (Secuvam).
Representative Product Drawing

INTENDED USE OF THE DEVICE
Intended Use
The "Leucoflex MTL1 Leukocyte Reduction Filter for Whole Blood" is intended for the pre-storage leukoreduction of whole blood initiated between 4 and 7 hours after collection if whole blood is stored at ambient temperature, or between 4 and 8 hours of storage at 1 to 6 C. The collection set provides for subsequent preparation of AS-1 Red Blood Cells, Leukocytes Reduced (adenine saline added) and Plasma, Leukocytes Reduced in a closed system.
The AS-1 Red Blood Cells, Leukocytes Reduced and Plasma, Leukocytes Reduced may then be stored for the maximum allowable dating periods.
WARNINGS SECTION
WARNING
- Avoid contact with sharp objects.
- DO NOT USE if the overwrap or blood bag system shows any signs of deterioration.
- DO NOT USE if the solutions are not clear.
- Dispose of all system components that have been contaminated with blood in a biohazard container as per your institution's SOP.
- Dispose of all sharps as per your institution's SOP.
PRECAUTIONS SECTION
CAUTIONS
- Do not fold or squeeze the Leucolex MTL1 filter. Inappropriate handling may adversely affect filtration.
- Check for kinks in the tubing prior to collection and filtration.
- Rx only.
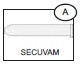
The protective shield for needle Secuvam (A) and the vacuum tube adapter Vacuvam (B) features of the collection set aid in the safe collection of vacuum tube samples from the sample diversion pouch Bactivam (C) and the disposal of donor needles after blood collection by providing protection from accidental needlestick.
All or part of this medical device is made of PVC plasticized with DEHP. According to some studies, DEHP could potentially be harmful to the reproductive system of male fetuses. The prescriber is solely responsible for choosing to use this device on women who are either pregnant or breast- feeding, or on young male infants. Nevertheless, DEHP-plasticized PVC is in compliance with the European Pharmacopeia.
Risks linked to the reuse: Septic risk.
All maunfacturing processes and all components in contact with the donors, the users and the blood components dedicated to the patients are not made with natural rubber latex.
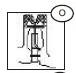
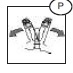
INSTRUCTIONS FOR USE SECTION
HANDLING OF PORT PROTECTORS
-
Using both hands tear the port protector as indicated. (O)
-
Hold down the port protector pieces with one hand. (P)
-
Using the free hand, take the spike and engage into the port. (Q)
-
Twist the spike to secure fully in port. (Q)