azithromycin dihydrate
These highlights do not include all the information needed to use AZITHROMYCIN TABLETS safely and effectively. See full prescribing information for AZITHROMYCIN TABLETS.AZITHROMYCIN tablets, 600 mg, for oral useInitial U.S. Approval: 1991
c9990a1d-5238-403e-9221-72a9314d7b5e
HUMAN PRESCRIPTION DRUG LABEL
Sep 16, 2025
Lupin Pharmaceuticals, Inc.
DUNS: 089153071
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
azithromycin dihydrate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Azithromycin Tablets USP, 600 mg
30 Tablets- Container Label
NDC 68180-863-06


DESCRIPTION SECTION
11 DESCRIPTION
Azithromycin tablets USP contain the active ingredient azithromycin, a macrolide antibacterial drug, for oral administration. Azithromycin has the chemical name (2R,3S,4R,5R,8R,10R,11R,12S,13S,14R)-13-[(2,6-dideoxy-3-C-methyl-3-O-methyl-α- L-ribo- hexopyranosyl)oxy]-2-ethyl-3,4,10-trihydroxy-3,5,6,8,10,12,14-heptamethyl-11-[[3,4,6-trideoxy-3-(dimethylamino)-β-D- xylo-hexopyranosyl]oxy]-1-oxa-6-azacyclopentadecan-15-one. Azithromycin is derived from erythromycin; however, it differs chemically from erythromycin in that a methyl-substituted nitrogen atom is incorporated into the lactone ring. Its molecular formula is C38H72N2O12, and its molecular weight is 749.0. Azithromycin has the following structural formula:
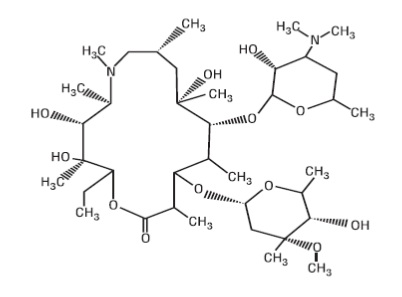
Azithromycin, as the dihydrate, is a white to almost white crystalline powder with a molecular formula of C38H72N2O12•2H2O and a molecular weight of 785.02
Azithromycin tablets USP contain azithromycin dihydrate equivalent to 600 mg azithromycin. They also contain the following inactive ingredients: croscarmellose sodium, dibasic calcium phosphate, hydroxypropyl methyl cellulose, lactose monohydrate, magnesium stearate, sodium lauryl sulfate, titanium dioxide and triacetin.
Organic Impurities Test Pending.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Azithromycin tablets may be taken with or without food. However, increased tolerability has been observed when tablets are taken with food.
Patients should also be cautioned not to take aluminum- and magnesium- containing antacids and azithromycin simultaneously.
The patient should be directed to discontinue azithromycin immediately and contact a physician if any signs of an allergic reaction occur.
Direct parents or caregivers to contact their physician if vomiting and irritability with feeding occurs in the infant.
Patients should be counseled that antibacterial drugs, including azithromycin, should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When azithromycin is prescribed to treat bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by azithromycin or other antibacterial drugs in the future.
Diarrhea is a common problem caused by antibacterial which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibacterials, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibacterial. If this occurs, patients should contact their physician as soon as possible.
LUPIN and the
 are registered trademarks of Lupin Pharmaceuticals, Inc.
are registered trademarks of Lupin Pharmaceuticals, Inc.
Manufactured for:
Lupin Pharmaceuticals, Inc.
Naples, FL 34108
United States
Manufactured by:
Lupin Limited
Goa - 403722
India
Revised: October 2024 ID#: 276179
