Nabumetone
NABUMETONE TABLETS USP Rx Only
06f73fa8-7a8f-4160-939e-a25980a11906
HUMAN PRESCRIPTION DRUG LABEL
Dec 1, 2023
Bryant Ranch Prepack
DUNS: 171714327
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Nabumetone
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Drug Labeling Information
DESCRIPTION SECTION
DESCRIPTION
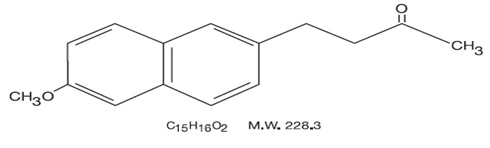
Nabumetone is a naphthylalkanone designated chemically as 4-(6-methoxy-2- naphthalenyl)-2- butanone. It has the following structure:
Nabumetone, USP is a white or almost white crystalline substance with a molecular weight of 228.3. It is nonacidic, freely soluble in acetone, sparingly soluble in alcohol and in methanol, practically insoluble in water. It has an n-octanol:phosphate buffer partition coefficient of 2400 at pH 7.4.
Each tablet, for oral administration contains either 500 mg or 750 mg of nabumetone, USP. In addition, each tablet contains the following inactive ingredients: hypromellose, microcrystalline cellulose, sodium lauryl sulfate, sodium starch glycolate, polyethylene glycol and titanium dioxide.
