Milia remover treatment
84735-009 Milia remover treatment
353b9e99-f839-24df-e063-6294a90a4725
HUMAN OTC DRUG LABEL
May 16, 2025
Hengyang Chuangjiujia Trading Co., Ltd.
DUNS: 977292157
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Dioctyl Dimethyl Ammonium Chloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (8)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
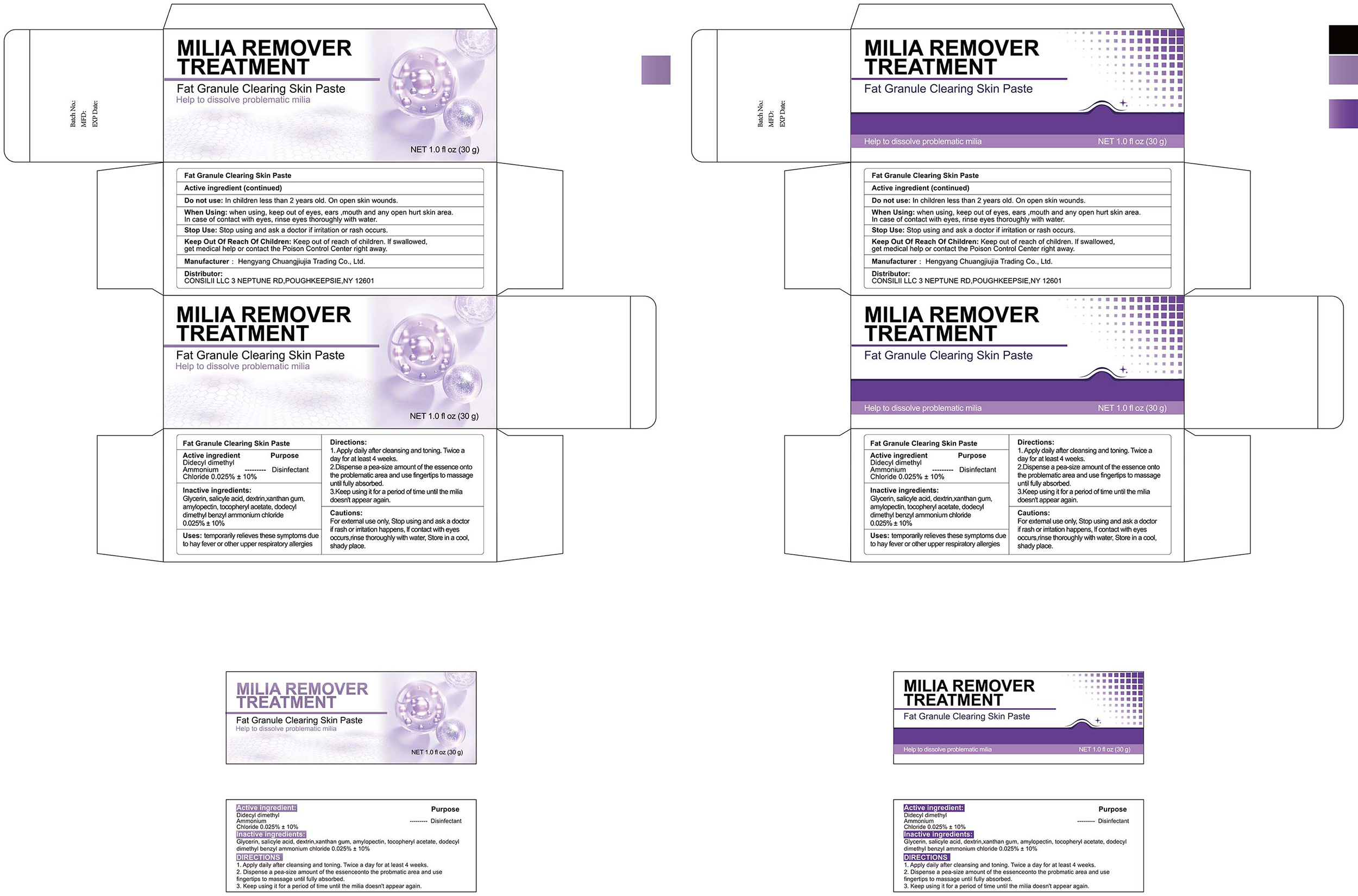
INDICATIONS & USAGE SECTION
Uses: temporarily relieves these symptoms dueto hay fever or other upper respiratory allergies
OTC - ACTIVE INGREDIENT SECTION
Dioctyl Dimethyl Ammonium Chloride 0.025%
OTC - PURPOSE SECTION
Disinfectant
WARNINGS SECTION
For extemal use only.
OTC - DO NOT USE SECTION
Do not use: In children less than 2 years old. On open skin wounds.
OTC - WHEN USING SECTION
When Using: when using, keep out of eyes, ears ,mouth and any open hurt skin area. In case of contact with eyes, rinse eyes thoroughly with water.
OTC - STOP USE SECTION
Stop Use: Stop using and ask a doctor if irritation or rash occurs.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep Out Of Reach Of Children: Keep out of reach of children. if swallowed
get medical help or contact the Poison Control Center right away.
DOSAGE & ADMINISTRATION SECTION
1.Apply daily after deansing and toning. Twice aday for at least 4 weeks.
2.Dispense a pea-size amount of the essence ontothe problematic area and use
fingertips to massageuntil fully absorbed
3.Keep using it for a perod of time until the miliadoesn't appear again.
INACTIVE INGREDIENT SECTION
Glycerin
Salicylic Acid
Dextrin
Xanthan Gum
Amylopectin
Tocopheryl Acetate
Dodecyl Dimethyl Benzyl Ammonium Chloride
