Excede
EXCEDE (Ceftiofur Crystalline Free Acid)Sterile Suspension
9922e056-dcb0-46fd-bb56-9863bb0a189e
PRESCRIPTION ANIMAL DRUG LABEL
Aug 13, 2025
Zoetis Inc.
DUNS: 828851555
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
ceftiofur
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (1)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 250 mL Vial label
250mL label
40052919

INDICATIONS & USAGE SECTION
INDICATION
EXCEDE Sterile Suspension is indicated for the treatment of lower respiratory tract infections in horses caused by susceptible strains of Streptococcus equi ssp. zooepidemicus.
CONTRAINDICATIONS SECTION
CONTRAINDICATIONS
EXCEDE Sterile Suspension is contraindicated in horses with known allergy to ceftiofur or to ß-lactam (penicillins and cephalosporins) group antimicrobials. Due to the extended exposure in horses, based on the drug's pharmacokinetic properties, adverse reactions may require prolonged care.
ADVERSE REACTIONS SECTION
ADVERSE REACTIONS
Pre-Approval Experience:
A total of 373 horses of various breeds, ranging in age from 4 months to 20
years, were included in the field study safety analysis. Adverse reactions
reported in horses treated with EXCEDE and the placebo control are summarized
in Table 2.
Injection site swelling (edema) was reported in 10 of 278 (3.6%) EXCEDE- treated horses and 1 of 95 (1 %) of the placebo-treated horses. Of the 10 EXCEDE-treated horses with injection site swelling, 8 horses had swellings of 4 cm or less in diameter, one horse had a 10 cm diameter swelling and one horse had injection site reactions to both injections measuring 25 x 12 cm each. The injection site reactions in EXCEDE treated horses resolved over 1 to 20 days.
At least one episode of diarrhea, loose, soft, or cowpie stools were observed in 25 of 278 (9%) of the EXCEDE-treated horses and 7 of 95 (7%) of the placebo-treated horses. The duration of episodes in EXCEDE-treated horses ranged from a single observation of loose stool to observations lasting 6 days. All cases were self-limiting and resolved with minimal (a single dose of loperamide) or no treatment.
Table 2. Number of Horses with Adverse Reactions During the Field Study with EXCEDE.|
Adverse Reaction |
EXCEDE (n=278) |
Placebo (n=95) |
|---|---|---|
|
Diarrhea/Soft Stool |
25 (9%) |
7 (7%) |
|
Injection Site Swelling |
10 (4%) |
1 (1%) |
Post Approval Experience (2019):
The following adverse events are based on post-approval adverse drug
experience reporting for Excede. Not all adverse events are reported to
FDA/CVM. It is not always possible to reliably estimate the adverse event
frequency or establish a causal relationship to product exposure using these
data.
The following adverse events for horses are listed in decreasing order by system and decreasing order within system classes.
Injection site reactions: swelling, pain, inflammation, infection, necrosis, muscle stiffness, fibrosis, injection site stiffness/ reluctance to move, hair change, lameness, granuloma.
Systemic: fever, lethargy, edema at locations other than injection site, anorexia.
Gastrointestinal: colic, diarrhea.
Neurologic: ataxia, muscle tremor, seizure, loss of consciousness.
Immune (Allergic reactions): anaphylaxis, urticaria, allergic edema (face, face and neck, lip, or limb edema).
In some cases, death has been reported as an outcome of the adverse events listed above. Sudden death (within minutes), or the immediate onset of seizures or collapse, followed by death or euthanasia, have been reported.
To report suspected adverse events, for technical assistance or to obtain a copy of the Safety Data Sheet (SDS) contact Zoetis, Inc. at (888) 963-8471. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at http://www.fda.gov/reportanimalae.
SPL UNCLASSIFIED SECTION
Approved by FDA under NADA # 141-209
zoetis
Distributed by:
Zoetis Inc.
Kalamazoo, MI 49007
https://www.zoetisus.com/products/cattle/excede
1-888-963-8471
Revised: May 2025
40052974
DESCRIPTION SECTION
DESCRIPTION
EXCEDE Sterile Suspension is a ready-to-use formulation that contains the crystalline free acid of ceftiofur, which is a broad spectrum cephalosporin antibiotic active against Gram-positive and Gram-negative bacteria including ß-lactamase-producing strains. Like other cephalosporins, ceftiofur is bactericidal, in vitro, resulting from inhibition of cell wall synthesis.
Each mL of this ready-to-use sterile suspension contains ceftiofur crystalline free acid equivalent to 200 mg ceftiofur, in a caprylic/capric triglyceride and cottonseed oil based suspension.
Figure 1. Structure of ceftiofur crystalline free acid:

Chemical name of ceftiofur crystalline free acid:
7-[[2-(2-Amino-4-thiazolyl)-2-(methoxyimino)acetyl]amino]- 3-[[(2-furanylcarbonyl)thio] methyl]-8-oxo-5-thia-1- azabicyclo[4.2.0]oct-2-ene 2-carboxylic acid
DOSAGE & ADMINISTRATION SECTION
DOSAGE AND ADMINISTRATION
Shake well before using.
Administer two intramuscular injections to horses, 4 days apart, at a dose of 3.0 mg/lb (6.6 mg/kg). A maximum of 20 mL per injection site may be administered. Therapeutic drug concentrations are maintained for 6 days after the second injection (or a total of 10 days from the beginning of treatment) against Streptococcus equi ssp. zooepidemicus.
Table 1. Dosing Schedule for EXCEDE Sterile Suspension.|
Weight |
Dose Volume |
Weight |
Dose Volume | |
|
100 |
1.5 |
1100 |
16.5 | |
|
200 |
3.0 |
1200 |
18.0 | |
|
300 |
4.5 |
1300 |
19.5 | |
|
400 |
6.0 |
1400 |
21.0 | |
|
500 |
7.5 |
1500 |
22.5 | |
|
600 |
9.0 |
1600 |
24.0 | |
|
700 |
10.5 |
1700 |
25.5 | |
|
800 |
12.0 |
1800 |
27.0 | |
|
900 |
13.5 |
1900 |
28.5 | |
|
1000 |
15.0 |
2000 |
30.0 |
WARNINGS SECTION
Warnings and Precautions
Human Safety Warnings
Not for use in humans. For use in animals only. Keep this and all drugs out of
reach of children.
Consult a physician in case of accidental human exposure. Penicillins and cephalosporins can cause allergic reactions in sensitized individuals. Topical exposure to such antimicrobials, including ceftiofur, may elicit mild to severe allergic reactions in some individuals. Repeated or prolonged exposure may lead to sensitization. Avoid direct contact of the product with the skin, eyes, mouth and clothing. Sensitization of the skin may be avoided by wearing protective gloves. Persons with a known sensitivity to penicillin or cephalosporins should avoid exposure to this product. In the case of accidental eye exposure, flush with water for 15 minutes. In case of accidental skin exposure, wash with soap and water. Remove contaminated clothing. If allergic reaction occurs (e.g. skin rash, hives, difficult breathing) seek medical attention.
RESIDUE WARNING SECTION
RESIDUE WARNINGS
*Following label use as either a single-dose or 2-dose regimen, a 13-day pre-slaughter withdrawal period is required after the last treatment.
*Following label use as either a single-dose or 2-dose regimen, no milk discard period is required for this product.
*Use of dosages in excess of 3.0 mg CE/lb (6.6 mg CE/kg) BW or administration by unapproved routes (subcutaneous injection in
the neck or intramuscular injection) may cause violative residues.
*A withdrawal period has not been established for this product in preruminating calves.
*Do not use in calves to be processed for veal.
PRECAUTIONS SECTION
Antibacterial Warnings
Use of antibacterial drugs in the absence of a susceptible bacterial infection is unlikely to provide benefit to treated animals and may increase the risk of the development of drug-resistant bacteria.
CLINICAL PHARMACOLOGY SECTION
CLINICAL PHARMACOLOGY
Ceftiofur is a beta-lactam antibiotic from the cephalosporin class. Beta lactams exert their inhibitory effect by interfering with bacterial cell wall synthesis. This interference is primarily due to its covalent binding to the penicillin-binding proteins, which are essential for synthesis of the bacterial wall. Ceftiofur administered as either ceftiofur sodium (NAXCEL® Sterile Powder) or ceftiofur crystalline free acid (EXCEDE Sterile Suspension) is rapidly metabolized to desfuroylceftiofur, the primary metabolite with antimicrobial activity. Two intramuscular injections of EXCEDE Sterile Suspension at a dose of 6.6 mg/kg body weight in the horse provide concentrations of ceftiofur and desfuroylceftiofur related metabolites in plasma above the therapeutic target of 0.2 µg/mL for the entire 96 hour (4 day) dosing interval and for 6 days after the second injection (or a total of 10 days from the beginning of treatment) (see Figure 2 and Table 3).
Figure 2. Average plasma concentration of ceftiofur and desfuroylceftiofur related metabolites in horses following the intramuscular administration of either EXCEDE Sterile Suspension at a dose of 3.0 mg/lb (6.6 mg/kg) administered twice at a 96 hour interval or NAXCEL Sterile Powder at a dose of 1.0 mg/lb (2.2 mg/kg BW) once daily for 10 consecutive days.
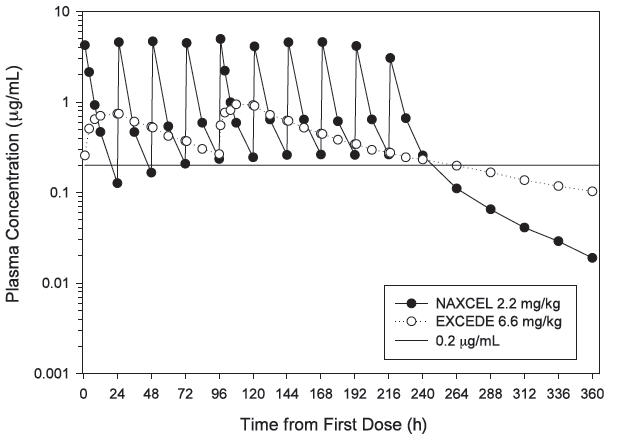
|
PK Parameter |
CCFA-SS at 6.6 mg/kg BW administered twice 96 h apart (Mean ± SD; n=12) |
Ceftiofur sodium at 2.2 mg/kg BW once daily for 10 days (Mean ± SD; n=11) | ||
|---|---|---|---|---|
|
AUC0-∞ |
157 (19.1) |
353 (44.9) | ||
|
t>0.2 (h) |
262 (29.0) |
ND | ||
|
Dose 1 |
Dose 2 |
Dose 1 |
Dose 10 | |
|
Tmax (h) |
21.6 (5.8) |
15.6 (6.3) |
1.0 |
2.0 (3.3) |
|
Cmax (µg/mL) |
0.78 (0.19) |
1.0 (0.24) |
4.31 ± 0.78 |
3.99 (1.23) |
MICROBIOLOGY
Ceftiofur is a cephalosporin antibiotic. Like other ß-lactam antimicrobials, ceftiofur exerts its inhibitory effect by interfering with bacterial cell wall synthesis. This interference is primarily due to its covalent binding to the penicillin-binding proteins (PBPs) (i.e., transpeptidase and carboxypeptidase), which are essential for synthesis of the bacterial wall. Ceftiofur is not active against Pseudomonas spp. and enterococci.
The minimum inhibitory concentration (MIC) values for ceftiofur against label- claim pathogens isolated from lower respiratory tract infections in horses enrolled in a 2007-2008 field effectiveness study are presented in Table 4. All MICs were determined in accordance with the Clinical and Laboratory Standards Institute (CLSI) standards.
Table 4. Activity of EXCEDE Against Pathogens Isolated from Horses Treated With EXCEDE in Field Studies in the U.S. During 2007-2008.|
Disease |
Pathogen |
Treatment Outcome |
of Isolates |
Time of Sample Collection |
MIC50 µg/mL |
MIC90 µg/mL |
MIC Range µg/mL |
|---|---|---|---|---|---|---|---|
| |||||||
|
Lower Respiratory Tract Infection |
Streptococcus equi ssp. zooepidemicus |
Success |
93* |
Pre-Treatment |
0.06 |
0.12 |
0.03-0.5 |
|
Failure |
42 |
Pre-Treatment |
0.06 |
0.25 |
0.03-0.5 |
STORAGE AND HANDLING SECTION
STORAGE CONDITIONS
Store at controlled room temperature 20° to 25°C (68° to 77°F). Shake well before using. Contents should be used within 12 weeks after the first dose is removed.
HOW SUPPLIED SECTION
HOW SUPPLIED
EXCEDE Sterile Suspension is available in the following package sizes:
100 mL vial
250 mL vial
