Azithromycin Dihydrate
These highlights do not include all the information needed to use AZITHROMYCIN TABLETS safely and effectively. See full prescribing information for AZITHROMYCIN TABLETS. AZITHROMYCIN tablets, for oral use Initial U.S. Approval: 1991
e9800bbe-e8bf-4689-a8a5-dc23b5d2ee56
HUMAN PRESCRIPTION DRUG LABEL
Mar 31, 2023
PD-Rx Pharmaceuticals, Inc.
DUNS: 156893695
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Azithromycin Dihydrate
PRODUCT DETAILS
INGREDIENTS (13)
Drug Labeling Information
INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
Azithromycin is a macrolide antibacterial drug indicated for the treatment of patients with mild to moderate infections caused by susceptible strains of the designated microorganisms in the specific conditions listed below. Recommended dosages and durations of therapy in adult and pediatric patient populations vary in these indications. [see Dosage and Administration (2)]
1.1 Adult Patients
- Acute bacterial exacerbations of chronic bronchitis due to Haemophilus influenzae, Moraxella catarrhalis, or Streptococcus pneumoniae.
- Acute bacterial sinusitis due to Haemophilus influenzae, Moraxella catarrhalis. or Streptococcus pneumoniae.
- Community-acquired pneumonia due to Chlamydophila pneumoniae, Haemophilus influenzae, Mycoplasma pneumoniae, or Streptococcus pneumoniae in patients appropriate for oral therapy.
- Pharyngitis/tonsillitis caused by Streptococcus pyogenes as an alternative to first-line therapy in individuals who cannot use first-line therapy.
- Uncomplicated skin and skin structure infections due to Staphylococcus aureus, Streptococcus pyogenes, or Streptococcus agalactiae.
- Urethritis and cervicitis due to Chlamydia trachomatis or Neisseria gonorrhoeae.
- Genital ulcer disease in men due to Haemophilus ducreyi (chancroid). Due to the small number of women included in clinical trials, the efficacy of azithromycin in the treatment of chancroid in women has not been established.
1.2 Pediatric Patients
[see Use in Specific Populations (8.4) and Clinical Studies (14.2)]
- Acute otitis media**(> 6 months of age)** caused by Haemophilus influenzae, Moraxella catarrhalis, or Streptococcus pneumoniae
- Community-acquired pneumonia**(> 6 months of age)** due to Chlamydophila pneumoniae, Haemophilus influenzae, Mycoplasma pneumonia, or Streptococcus pneumoniae in patients appropriate for oral therapy.
- Pharyngitis/tonsillitis**(> 2 years of age)** caused by Streptococcus pyogenes as an alternative to first-line therapy in individuals who cannot use first-line therapy.
1.3 Limitations of Use
Azithromycin should not be used in patients with pneumonia who are judged to be inappropriate for oral therapy because of moderate to severe illness or risk factors such as any of the following:
- patients with cystic fibrosis,
- patients with nosocomial infections,
- patients with known or suspected bacteremia,
- patients requiring hospitalization,
- elderly or debilitated patients, or
- patients with significant underlying health problems that may compromise their ability to respond to their illness (including immunodeficiency or functional asplenia).
1.4 Usage
To reduce the development of drug-resistant bacteria and maintain the effectiveness of azithromycin and other antibacterial drugs, azithromycin should be used only to treat infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Azithromycin is a macrolide antibacterial drug indicated for mild to moderate infections caused by designated, susceptible bacteria:
- Acute bacterial exacerbations of chronic bronchitis in adults ( 1.1)
- Acute bacterial sinusitis in adults ( 1.1)
- Uncomplicated skin and skin structure infections in adults ( 1.1)
- Urethritis and cervicitis in adults ( 1.1)
- Genital ulcer disease in men ( 1.1)
- Acute otitis media in pediatric patients (6 months of age and older) ( 1.2)
- Community-acquired pneumonia in adults and pediatric patients (6 months of age and older) ( 1.1, 1.2)
- Pharyngitis/tonsillitis in adults and pediatric patients (2 years of age and older) ( 1.1, 1.2)
Limitation of Use:
Azithromycin should not be used in patients with pneumonia who are judged to be inappropriate for oral therapy because of moderate to severe illness or risk factors. ( 1.3)
To reduce the development of drug-resistant bacteria and maintain the effectiveness of azithromycin and other antibacterial drugs, azithromycin should be used only to treat infections that are proven or strongly suspected to be caused by susceptible bacteria. ( 1.4)
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity
Serious allergic reactions, including angioedema, anaphylaxis, and dermatologic reactions including Acute Generalized Exanthematous Pustulosis (AGEP), Stevens-Johnson syndrome, and toxic epidermal necrolysis have been reported in patients on azithromycin therapy. [see Contraindications (4.1)]
Fatalities have been reported. Cases of Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) have also been reported. Despite initially successful symptomatic treatment of the allergic symptoms, when symptomatic therapy was discontinued, the allergic symptoms recurred soon thereafter in some patients without further azithromycin exposure**.** These patients required prolonged periods of observation and symptomatic treatment. The relationship of these episodes to the long tissue half-life of azithromycin and subsequent prolonged exposure to antigen is presently unknown.
If an allergic reaction occurs, the drug should be discontinued and appropriate therapy should be instituted. Physicians should be aware that allergic symptoms may reappear when symptomatic therapy has been discontinued.
5.2 Hepatotoxicity
Abnormal liver function, hepatitis, cholestatic jaundice, hepatic necrosis, and hepatic failure have been reported, some of which have resulted in death. Discontinue azithromycin immediately if signs and symptoms of hepatitis occur.
5.3 Infantile Hypertrophic Pyloric Stenosis (IHPS)
Following the use of azithromycin in neonates (treatment up to 42 days of life), IHPS has been reported. Direct parents and caregivers to contact their physician if vomiting or irritability with feeding occurs.
5.4 QT Prolongation
Prolonged cardiac repolarization and QT interval, imparting a risk of developing cardiac arrhythmia and torsades de pointes, have been seen with treatment with macrolides, including azithromycin. Cases of torsades de pointes have been spontaneously reported during postmarketing surveillance in patients receiving azithromycin. Providers should consider the risk of QT prolongation which can be fatal when weighing the risks and benefits of azithromycin for at-risk groups including:
- patients with known prolongation of the QT interval, a history of torsades de pointes, congenital long QT syndrome, bradyarrhythmias or uncompensated heart failure
- patients on drugs known to prolong the QT interval
- patients with ongoing proarrhythmic conditions such as uncorrected hypokalemia or hypomagnesemia, clinically significant bradycardia, and in patients receiving Class IA (quinidine, procainamide) or Class III (dofetilide, amiodarone, sotalol) antiarrhythmic agents.
Elderly patients may be more susceptible to drug-associated effects on the QT interval.
5.5 Clostridium difficile-Associated Diarrhea (CDAD)
Clostridium difficile-associated diarrhea has been reported with use of nearly all antibacterial agents, including azithromycin, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon, leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antibacterial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
5.6 Exacerbation of Myasthenia Gravis
Exacerbation of symptoms of myasthenia gravis and new onset of myasthenic syndrome have been reported in patients receiving azithromycin therapy.
5.7 Use in Sexually Transmitted Infections
Azithromycin, at the recommended dose, should not be relied upon to treat syphilis. Antibacterial agents used to treat non-gonococcal urethritis may mask or delay the symptoms of incubating syphilis. All patients with sexually transmitted urethritis or cervicitis should have a serologic test for syphilis and appropriate testing for gonorrhea performed at the time of diagnosis. Appropriate antibacterial therapy and follow-up tests for these diseases should be initiated if infection is confirmed.
5.8 Development of Drug-Resistant Bacteria
Prescribing azithromycin in the absence of a proven or strongly suspected bacterial infection is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
- Serious (including fatal) allergic and skin reactions: Discontinue azithromycin if reaction occurs. ( 5.1)
- Hepatotoxicity: Severe, and sometimes fatal, hepatotoxicity has been reported. Discontinue azithromycin immediately if signs and symptoms of hepatitis occur. ( 5.2)
- Infantile Hypertrophic Pyloric Stenosis (IHPS): Following the use of azithromycin in neonates (treatment up to 42 days of life), IHPS has been reported. Direct parents and caregivers to contact their physician if vomiting or irritability with feeding occurs. ( 5.3)
- Prolongation of QT interval and cases of torsades de pointes have been reported. This risk which can be fatal should be considered in patients with certain cardiovascular disorders including known QT prolongation or history torsades de pointes, those with proarrhythmic conditions, and with other drugs that prolong the QT interval. ( 5.4)
- Clostridium difficile-Associated Diarrhea: Evaluate patients if diarrhea occurs. ( 5.5)
- Azithromycin may exacerbate muscle weakness in persons with myasthenia gravis. ( 5.6)
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Nelfinavir
Co-administration of nelfinavir at steady-state with a single oral dose of azithromycin resulted in increased azithromycin serum concentrations. Although a dose adjustment of azithromycin is not recommended when administered in combination with nelfinavir, close monitoring for known adverse reactions of azithromycin, such as liver enzyme abnormalities and hearing impairment, is warranted. [see Adverse Reactions (6)]
7.2 Warfarin
Spontaneous postmarketing reports suggest that concomitant administration of azithromycin may potentiate the effects of oral anticoagulants such as warfarin, although the prothrombin time was not affected in the dedicated drug interaction study with azithromycin and warfarin. Prothrombin times should be carefully monitored while patients are receiving azithromycin and oral anticoagulants concomitantly.
7.3 Potential Drug-Drug Interactions with Macrolides
Interactions with digoxin, colchicine or phenytoin have not been reported in clinical trials with azithromycin. No specific drug interaction studies have been performed to evaluate potential drug-drug interactions. However, drug interactions have been observed with other macrolide products. Until further data are developed regarding drug interactions when digoxin, colchicine or phenytoin are used with azithromycin careful monitoring of patients is advised.
- Nelfinavir: Close monitoring for known adverse reactions of azithromycin, such as liver enzyme abnormalities and hearing impairment, is warranted. ( 7.1)
- Warfarin: Use with azithromycin may increase coagulation times; monitor prothrombin time. ( 7.2)
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data from published literature and postmarketing experience over several decades with azithromycin use in pregnant women have not identified any drug-associated risks for major birth defects, miscarriage, or adverse maternal or fetal outcomes (see Data) . Developmental toxicity studies with azithromycin in rats, mice, and rabbits showed no drug-induced fetal malformations at doses up to 4, 2, and 2 times, respectively, an adult human daily dose of 500 mg based on body surface area. Decreased viability and delayed development were observed in the offspring of pregnant rats administered azithromycin from day 6 of pregnancy through weaning at a dose equivalent to 4 times an adult human daily dose of 500 mg based on body surface area (see Data) .
The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Human Data
Available data from published observational studies, case series, and case reports over several decades do not suggest an increased risk for major birth defects, miscarriage, or adverse maternal or fetal outcomes with azithromycin use in pregnant women. Limitations of these data include the lack of randomization and inability to control for confounders such as underlying maternal disease and maternal use of concomitant medications.
Animal Data
Azithromycin administered during the period of organogenesis did not cause fetal malformations in rats and mice at oral doses up to 200 mg/kg/day (moderately maternally toxic). Based on body surface area, this dose is approximately 4 (rats) and 2 (mice) times an adult human daily dose of 500 mg. In rabbits administered azithromycin at oral doses of 10 mg/kg/day, 20 mg/kg/day, and 40 mg/kg/day during organogenesis, reduced maternal body weight and food consumption were observed in all groups; no evidence of fetotoxicity or teratogenicity was observed at these doses, the highest of which is estimated to be 2 times an adult human daily dose of 500 mg based on body surface area.
In a pre- and postnatal development study, azithromycin was administered orally to pregnant rats from day 6 of pregnancy until weaning at doses of 50 mg/kg/day or 200 mg/kg/day. Maternal toxicity (reduced food consumption and body weight gain; increased stress at parturition) was observed at the higher dose. Effects in the offspring were noted at 200 mg/kg/day during the postnatal development period (decreased viability, delayed developmental landmarks). These effects were not observed in a pre- and postnatal rat study when up to 200 mg/kg/day of azithromycin was given orally beginning on day 15 of pregnancy until weaning.
8.2 Lactation
Risk Summary
Azithromycin is present in human milk (see Data) . Non-serious adverse reactions have been reported in breastfed infants after maternal administration of azithromycin (see Clinical Considerations) . There are no available data on the effects of azithromycin on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for azithromycin and any potential adverse effects on the breastfed infant from azithromycin or from the underlying maternal condition.
Clinical Considerations
Advise women to monitor the breastfed infant for diarrhea, vomiting, or rash.
Data
Azithromycin breastmilk concentrations were measured in 20 women after receiving a single 2 g oral dose of azithromycin during labor. Breastmilk samples collected on days 3 and 6 postpartum as well as 2 and 4 weeks postpartum revealed the presence of azithromycin in breastmilk up to 4 weeks after dosing. In another study, a single dose of azithromycin 500 mg was administered intravenously to 8 women prior to incision for cesarean section. Breastmilk (colostrum) samples obtained between 12 and 48 hours after dosing revealed that azithromycin persisted in breastmilk up to 48 hours.
8.4 Pediatric Use
[see Clinical Pharmacology (12.3), Indications and Usage (1.2), and Dosage and Administration (2.2)]
Safety and effectiveness in the treatment of pediatric patients with acute otitis media, acute bacterial sinusitis and community-acquired pneumonia under 6 months of age have not been established. Use of azithromycin for the treatment of acute bacterial sinusitis and community-acquired pneumonia in pediatric patients (6 months of age or greater) is supported by adequate and well-controlled trials in adults.
Pharyngitis/Tonsillitis: Safety and effectiveness in the treatment of pediatric patients with pharyngitis/tonsillitis under 2 years of age have not been established.
8.5 Geriatric Use
In multiple-dose clinical trials of oral azithromycin, 9% of patients were at least 65 years of age (458/4,949) and 3% of patients (144/4,949) were at least 75 years of age. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in response between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Elderly patients may be more susceptible to development of torsades de pointes arrhythmias than younger patients. [see Warnings and Precautions (5.4)]
- Pediatric use: Safety and effectiveness in the treatment of patients under 6 months of age have not been established. ( 8.4)
- Geriatric use: Elderly patients may be more susceptible to development of torsades de pointes arrhythmias. ( 8.5)
DESCRIPTION SECTION
11 DESCRIPTION
Azithromycin Tablets, USP contain the active ingredient azithromycin, a macrolide antibacterial drug, for oral administration. Azithromycin has the chemical name (2R,3S,4R,5R,8R,10R,11R,12S,13S,14R)-13-[(2,6-dideoxy-3-C-methyl-3-O-methyl-α- L-ribo- hexopyranosyl)oxy]-2-ethyl-3,4,10-trihydroxy-3,5,6,8,10,12,14-heptamethyl-11-[[3,4,6-trideoxy-3-(dimethylamino)-β-D- xylo-hexopyranos yl]oxy]-1-oxa-6-azacyclopentadecan-15-one. Azithromycin is derived from erythromycin; however, it differs chemically from erythromycin in that a methyl-substituted nitrogen atom is incorporated into the lactone ring. Its molecular formula is C 38H 72N 2O 12, and its molecular weight is 749.00. Azithromycin has the following structural formula:
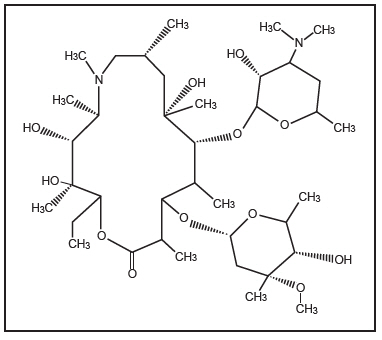
Azithromycin, as the dihydrate, is a white crystalline powder with a molecular formula of C 38H 72N 2O 12∙2H 2O and a molecular weight of 785.0.
Azithromycin is supplied as tablets containing azithromycin dihydrate equivalent to 250 mg azithromycin and the following inactive ingredients: croscarmellose sodium, dibasic calcium phosphate anhydrous, FD&C Blue #1 aluminum lake and lecithin, FD&C Red #40 aluminum Lake, FD&C Yellow #6 aluminum Lake, macrogol/PEG, magnesium stearate, polyvinyl alcohol, pregelatinized starch, talc, and titanium dioxide.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals have not been performed to evaluate carcinogenic potential. Azithromycin has shown no mutagenic potential in standard laboratory tests: mouse lymphoma assay, human lymphocyte clastogenic assay, and mouse bone marrow clastogenic assay. In fertility studies conducted in male and female rats, oral administration of azithromycin for 64 to 66 days (males) or 15 days (females) prior to and during cohabitation resulted in decreased pregnancy rate at 20 mg/kg/day and 30 mg/kg/day when both males and females were treated with azithromycin. This minimal effect on pregnancy rate (approximately 12% reduction compared to concurrent controls) did not become more pronounced when the dose was increased from 20 mg/kg/day to 30 mg/kg/day (approximately 0.4 to 0.6 times the adult daily dose of 500 mg based on body surface area) and it was not observed when only one animal in the mated pair was treated. There were no effects on any other reproductive parameters, and there were no effects on fertility at 10 mg/kg/day. The relevance of these findings to patients being treated with azithromycin at the doses and durations recommended in the prescribing information is uncertain.
13.2 Animal Toxicology and/or Pharmacology
Phospholipidosis (intracellular phospholipid accumulation) has been observed in some tissues of mice, rats, and dogs given multiple doses of azithromycin. It has been demonstrated in numerous organ systems (e.g., eye, dorsal root ganglia, liver, gallbladder, kidney, spleen, and/or pancreas) in dogs and rats treated with azithromycin at doses which, expressed on the basis of body surface area, are similar to or less than the highest recommended adult human dose. This effect has been shown to be reversible after cessation of azithromycin treatment. Based on the pharmacokinetic data, phospholipidosis has been seen in the rat (50 mg/kg/day dose) at the observed maximal plasma concentration of 1.3 mcg/mL (1.6 times the observed C max of 0.821 mcg/mL at the adult dose of 2 g). Similarly, it has been shown in the dog (10 mg/kg/day dose) at the observed maximal serum concentration of 1 mcg/mL (1.2 times the observed C max of 0.821 mcg/mL at the adult dose of 2 g). Phospholipidosis was also observed in neonatal rats dosed for 18 days at 30 mg/kg/day, which is less than the pediatric dose of 60 mg/kg based on the surface area. It was not observed in neonatal rats treated for 10 days at 40 mg/kg/day with mean maximal serum concentrations of 1.86 mcg/mL, approximately 1.5 times the C max of 1.27 mcg/mL at the pediatric dose. Phospholipidosis has been observed in neonatal dogs (10 mg/kg/day) at maximum mean whole blood concentrations of 3.54 mcg/mL, approximately 3 times the pediatric dose C max. The significance of these findings for animals and for humans is unknown.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Azithromycin Tablets USP, 250 mg are supplied as red, oval, film coated tablets containing azithromycin dihydrate equivalent to 250 mg of azithromycin.
Azithromycin Tablets USP, 250 mg are debossed "OE" on one side and "250" on the other side. These are packaged in bottles as follows:
Bottles of 2 tablets NDC 72789-081-02
Bottles of 4 tablets NDC 72789-081-04
Store at 20° to 25°C (68° to 77°F); excursions permitted from 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Dispense in tight containers (USP).
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
General Patient Counseling
Azithromycin tablets can be taken with or without food.
Patients should also be cautioned not to take aluminum- and magnesium- containing antacids and azithromycin simultaneously.
The patient should be directed to discontinue azithromycin immediately and contact a physician if any signs of an allergic reaction occur.
Direct parents or caregivers to contact their physician if vomiting and irritability with feeding occurs in the infant.
Patients should be counseled that antibacterial drugs including azithromycin should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When azithromycin is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of the therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by azithromycin or other antibacterial drugs in the future.
Diarrhea is a common problem caused by antibacterials which usually ends when the antibacterial is discontinued. Sometimes after starting treatment with antibacterials patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibacterial drug. If this occurs, patients should contact their physician as soon as possible.
See****FDA-approved Patient Labeling
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Adult Patients
[see Indications and Usage (1.1) and Clinical Pharmacology (12.3)]
|
Infection * |
Recommended Dose/Duration of Therapy |
|---|---|
| |
|
Community-acquired pneumonia |
500 mg as a single dose on Day 1, followed by 250 mg once daily on Days 2 through 5 |
|
Acute bacterial exacerbations of chronic obstructive pulmonary disease |
500 mg once daily for 3 days |
|
Acute bacterial sinusitis |
500 mg once daily for 3 days |
|
Genital ulcer disease (chancroid) |
One single 1 gram dose |
|
Non-gonococcal urethritis and cervicitis |
One single 1 gram dose |
|
Gonococcal urethritis and cervicitis |
One single 2 gram dose |
Azithromycin tablets can be taken with or without food.
2.2 Pediatric Patients 1
|
Infection * |
Recommended Dose/Duration of Therapy |
|---|---|
|
1 see dosing tables below for maximum doses evaluated by indication | |
| |
|
Acute otitis media |
30 mg/kg as a single dose or 10 mg/kg once daily for 3 days or 10 mg/kg as a single dose on Day 1 followed by 5 mg/kg/day on Days 2 through 5 |
|
Acute bacterial sinusitis |
10 mg/kg once daily for 3 days |
|
Community-acquired pneumonia |
10 mg/kg as a single dose on Day 1 followed by 5 mg/kg once daily on Days 2 through 5 |
|
Pharyngitis/tonsillitis |
12 mg/kg once daily for 5 days |
Azithromycin for oral suspension can be taken with or without food.
PEDIATRIC DOSAGE GUIDELINES FOR OTITIS MEDIA, ACUTE BACTERIAL SINUSITIS, AND COMMUNITY-ACQUIRED PNEUMONIA
**(Age 6 months and above,[seeUse in Specific Populations (8.4)****] **)
Based on Body Weight
OTITIS MEDIA AND COMMUNITY-ACQUIRED PNEUMONIA: (5-Day Regimen) *|
Dosing Calculated on 10 mg/kg/day Day 1 and 5 mg/kg/day Days 2 to 5. | ||||||
|---|---|---|---|---|---|---|
|
Weight |
100 mg/5 mL |
200 mg/5 mL |
Total mL per Treatment Course |
Total mg per Treatment Course | ||
|
Kg |
Day 1 |
Days 2 to 5 |
Day 1 |
Days 2 to 5 | ||
| ||||||
|
5 |
2.5 mL; |
1.25mL; |
7.5 mL |
150 mg | ||
|
10 |
5 mL; |
2.5 mL; |
15 mL |
300 mg | ||
|
20 |
5 mL; |
2.5 mL; |
15 mL |
600 mg | ||
|
30 |
7.5 mL; |
3.75 mL; |
22.5 mL |
900 mg | ||
|
40 |
10 mL; |
5 mL; |
30 mL |
1,200 mg | ||
|
50 and above |
12.5 mL; |
6.25 mL; |
37.5 mL |
1,500 mg |
|
Dosing Calculated on 10 mg/kg/day. | ||||
|---|---|---|---|---|
|
Weight |
100 mg/5 mL |
200 mg/5 mL |
Total mL per Treatment Course |
Total mg per Treatment Course |
|
Kg |
Days 1 to 3 |
Days 1 to 3 | ||
| ||||
|
5 |
2.5 mL; (½ tsp) |
7.5 mL |
150 mg | |
|
10 |
5 mL; (1 tsp) |
15 mL |
300 mg | |
|
20 |
5 mL; (1 tsp) |
15 mL |
600 mg | |
|
30 |
7.5 mL; (1½ tsp) |
22.5 mL |
900 mg | |
|
40 |
10 mL; (2 tsp) |
30 mL |
1,200 mg | |
|
50 and above |
12.5 mL; (2½ tsp) |
37.5 mL |
1,500 mg |
|
Dosing Calculated on 30 mg/kg as a single dose. | |||
|---|---|---|---|
|
Weight |
200 mg/5 mL |
Total mL per Treatment Course |
Total mg per Treatment Course |
|
Kg |
1-Day Regimen | ||
|
5 |
3.75 mL; (3/4 tsp) |
3.75 mL |
150 mg |
|
10 |
7.5 mL; (1½ tsp) |
7.5 mL |
300 mg |
|
20 |
15 mL; (3 tsp) |
15 mL |
600 mg |
|
30 |
22.5 mL; (4½ tsp) |
22.5 mL |
900 mg |
|
40 |
30 mL; (6 tsp) |
30 mL |
1,200 mg |
|
50 and above |
37.5 mL; (7½ tsp) |
37.5 mL |
1,500 mg |
The safety of re-dosing azithromycin in pediatric patients who vomit after receiving 30 mg/kg as a single dose has not been established. In clinical studies involving 487 patients with acute otitis media given a single 30 mg/kg dose of azithromycin, 8 patients who vomited within 30 minutes of dosing were re-dosed at the same total dose.
Pharyngitis/Tonsillitis: The recommended dose of azithromycin for children with pharyngitis/tonsillitis is 12 mg/kg once daily for 5 days. (See chart below.)
PEDIATRIC DOSAGE GUIDELINES FOR PHARYNGITIS/TONSILLITIS
**(Age 2 years and above,[seeUse in Specific Populations (8.4)****] **)
Based on Body Weight
PHARYNGITIS/TONSILLITIS: (5-Day Regimen)|
Dosing Calculated on 12 mg/kg/day for 5 days. | |||
|---|---|---|---|
|
Weight |
200 mg/5 mL |
Total mL per Treatment Course |
Total mg per Treatment Course |
|
Kg |
Days 1 to 5 | ||
|
8 |
2.5 mL; (½ tsp) |
12.5 mL |
500 mg |
|
17 |
5 mL; (1 tsp) |
25 mL |
1,000 mg |
|
25 |
7.5 mL; (1½ tsp) |
37.5 mL |
1,500 mg |
|
33 |
10 mL; (2 tsp) |
50 mL |
2,000 mg |
|
40 |
12.5 mL; (2½ tsp) |
62.5 mL |
2,500 mg |
*Adult Patients (2.1)
|
Infection |
Recommended Dose/Duration of Therapy |
|---|---|
|
Community-acquired pneumonia (mild severity) |
500 mg as a single dose on Day 1, followed by 250 mg once daily on Days 2 through 5 |
|
Acute bacterial exacerbations of chronic bronchitis (mild to moderate) |
500 mg as a single dose on Day 1, followed by 250 mg once daily on Days 2 through 5 or 500 mg once daily for 3 days |
|
Acute bacterial sinusitis |
500 mg once daily for 3 days |
|
Genital ulcer disease (chancroid) |
One single 1 gram dose |
|
Gonococcal urethritis and cervicitis |
One single 2 gram dose |
*Pediatric Patients (2.2)
|
Infection |
Recommended Dose/Duration of Therapy |
|---|---|
|
Acute otitis media (6 months of age and older) |
30 mg/kg as a single dose or 10 mg/kg once daily for 3 days or 10 mg/kg as a single dose on Day 1 followed by 5 mg/kg/day on Days 2 through 5 |
|
Acute bacterial sinusitis (6 months of age and older) |
10 mg/kg once daily for 3 days |
|
Community-acquired pneumonia |
10 mg/kg as a single dose on Day 1 followed by 5 mg/kg once daily on Days 2 through 5 |
|
Pharyngitis/tonsillitis (2 years of age and older) |
12 mg/kg once daily for 5 days |
