ZONISADE
These highlights do not include all the information needed to use ZONISADE safely and effectively. See full prescribing information for ZONISADE ZONISADE (zonisamide oral suspension)Initial U.S. Approval: 2000
ac16fa15-32e9-4f92-8bc6-d8d41ae002c6
HUMAN PRESCRIPTION DRUG LABEL
Mar 30, 2023
Azurity Phramaceuticals, Inc.
DUNS: 117505635
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
ZONISAMIDE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - Carton Label
Carton Label
NDC 52652-8001-1
ZONISADE**®******
****(zonisamide
oral suspension)
100 mg/5 mL
FOR ORAL USE ONLY
SHAKE WELL BEFORE USE
150 mL Rx Only
ATTENTION PHARMACIST:
Dispense Medication Guide to each
patient. Medication Guide available at:
zonisade.com/medication-guide.pdf
azurity**®******
****pharmaceuticals
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Potentially Fatal Reactions to Sulfonamides
Fatalities have occurred as a result of severe reactions to sulfonamides (zonisamide is a sulfonamide) including Stevens-Johnson syndrome, toxic epidermal necrolysis, fulminant hepatic necrosis, agranulocytosis, aplastic anemia, and other blood dyscrasias [see Warnings and Precautions (5.2, 5.3, 5.4)]. Such reactions may occur when a sulfonamide is readministered irrespective of the route of administration. If signs of hypersensitivity or other serious reactions occur, discontinue ZONISADE immediately. Specific experience with sulfonamide-type adverse reaction to zonisamide is described below.
5.2 Serious Skin Reactions
Seven deaths from severe rash [i.e., Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN)] were reported in the first 11 years of marketing in Japan. All of the patients were receiving other drugs in addition to zonisamide. In postmarketing experience from Japan, a total of 49 cases of SJS or TEN have been reported, a reporting rate of 46 per million patient-years of exposure. Although this rate is greater than background, it is probably an underestimate of the true incidence because of under-reporting. There were no confirmed cases of SJS or TEN in the US, European, or Japanese development programs.
In the US and European randomized controlled trials [see Clinical Studies (14)], 6 of 269 (2.2%) patients who received zonisamide discontinued treatment because of rash compared to no patients who received placebo. Across all trials during the US and European development, rash that led to discontinuation of zonisamide was reported in 1.4% of patients (12.0 events per 1000 patient-years of exposure). During Japanese development, serious rash or rash that led to discontinuation of zonisamide was reported in 2.0% of patients (27.8 events per 1000 patient-years). Rash usually occurred early in treatment, with 85% reported within 16 weeks in the US and European studies and 90% reported within two weeks in the Japanese studies. There was no apparent relationship of dose to the occurrence of rash.
Discontinue ZONISADE at the first sign of rash, unless the rash is clearly not drug-related. If signs or symptoms suggest SJS/TEN, use of ZONISADE should not be resumed and alternative therapy should be considered.
5.3 Serious Hematologic Events
Two confirmed cases of aplastic anemia and one confirmed case of agranulocytosis were reported in the first 11 years of marketing in Japan, rates greater than generally accepted background rates. There were no cases of aplastic anemia and two confirmed cases of agranulocytosis in the US, European, or Japanese development programs. There is inadequate information to assess the relationship, if any, between dose and duration of treatment and these events.
5.4 Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multi-
Organ Hypersensitivity
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS), also known as multi-organ hypersensitivity, has occurred with zonisamide, the active ingredient in ZONISADE. Some of these events have been fatal or life- threatening. DRESS typically, although not exclusively, presents with fever, rash, lymphadenopathy and/or facial swelling, in association with other organ system involvement, such as hepatitis, nephritis, hematologic abnormalities, myocarditis, or myositis, sometimes resembling an acute viral infection. Eosinophilia is often present. This disorder is variable in its expression, and other organ systems not noted here may be involved. It is important to note that early manifestations of hypersensitivity (e.g., fever, lymphadenopathy) may be present even though rash is not evident.
If such signs or symptoms are present, the patient should be evaluated immediately. ZONISADE should be discontinued if an alternative etiology for the signs or symptoms cannot be established.
5.5 Oligohidrosis and Hyperthermia in Pediatric Patients
ZONISADE is not approved for use in patients below 16 years of age.
Oligohidrosis, sometimes resulting in heat stroke and hospitalization, is seen in association with zonisamide in pediatric patients.
During the pre-approval development program in Japan, one case of oligohidrosis was reported in 403 pediatric patients, an incidence of 1 case per 285 patient-years of exposure. While there were no cases reported in the US or European development programs, fewer than 100 pediatric patients participated in these trials.
In the first 11 years of marketing in Japan, 38 cases were reported, an estimated reporting rate of about 1 case per 10,000 patient-years of exposure. In the first year of marketing in the US, 2 cases were reported, an estimated reporting rate of about 12 cases per 10,000 patient-years of exposure. These rates are underestimates of the true incidence because of under-reporting. There has also been one report of heat stroke in an 18-year-old patient in the US.
Decreased sweating and an elevation in body temperature above normal characterized these cases. Many cases were reported after exposure to elevated environmental temperatures. Heat stroke, requiring hospitalization, was diagnosed in some cases.
Pediatric patients appear to be at an increased risk for zonisamide-associated oligohidrosis and hyperthermia. Patients, especially pediatric patients, treated with ZONISADE should be monitored closely for evidence of decreased sweating and increased body temperature, especially in warm or hot weather. Caution should be used when ZONISADE is prescribed with other drugs that predispose patients to heat-related disorders; these drugs include, but are not limited to, carbonic anhydrase inhibitors and drugs with anticholinergic activity.
5.6 Acute Myopia and Secondary Angle Closure Glaucoma
Acute myopia and secondary angle closure glaucoma have been reported in patients receiving zonisamide, the active ingredient in ZONISADE. Elevated intraocular pressure can lead to serious sequelae, including permanent vision loss, if left untreated.
Symptoms in reported cases have included acute onset of decreased visual acuity and/or ocular pain. Ophthalmologic findings can include myopia, anterior chamber shallowing, ocular hyperemia (redness), and increased intraocular pressure. Mydriasis may or may not be present. This syndrome may be associated with ciliochoroidal effusion resulting in anterior displacement of the lens and iris, with secondary angle closure glaucoma. Symptoms typically occur within one month after initiating zonisamide therapy. In contrast to primary narrow angle glaucoma, which is rare under 40 years of age, secondary angle closure glaucoma associated with zonisamide has been reported both in pediatric patients and in adults. ZONISADE is not approved for use in patients below 16 years of age. The primary treatment to reverse symptoms is discontinuation of ZONISADE as rapidly as possible, according to the judgment of the treating physician. Other therapeutic measures, in conjunction with discontinuation of ZONISADE, may be helpful. Myopia and secondary angle closure glaucoma usually resolve or improve after discontinuation of zonisamide.
5.7 Suicidal Behavior and Ideation
Antiepileptic drugs (AEDs), including ZONISADE, increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. Patients treated with any AED for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior.
Pooled analyses of 199 placebo-controlled clinical trials (mono- and adjunctive therapy) of 11 different AEDs showed that patients randomized to one of the AEDs had approximately twice the risk (adjusted Relative Risk 1.8, 95% CI:1.2, 2.7) of suicidal thinking or behavior compared to patients randomized to placebo. In these trials, which had a median treatment duration of 12 weeks, the estimated incidence rate of suicidal behavior or ideation among 27,863 AED-treated patients was 0.43%, compared to 0.24% among 16,029 placebo-treated patients, representing an increase of approximately one case of suicidal thinking or behavior for every 530 patients treated. There were four suicides in drug-treated patients in the trials and none in placebo- treated patients, but the number is too small to allow any conclusion about drug effect on suicide.
The increased risk of suicidal thoughts or behavior with AEDs was observed as early as one week after starting drug treatment with AEDs and persisted for the duration of treatment assessed. Because most trials included in the analysis did not extend beyond 24 weeks, the risk of suicidal thoughts or behavior beyond 24 weeks could not be assessed.
The risk of suicidal thoughts or behavior was generally consistent among drugs in the data analyzed. The finding of increased risk with AEDs of varying mechanisms of action and across a range of indications suggests that the risk applies to all AEDs used for any indication. The risk did not vary substantially by age (5-100 years) in the clinical trials analyzed.
Table 1 shows absolute and relative risk by indication for all evaluated AEDs.
Table 1. Risk by Indication for Antiepileptic Drugs in the Pooled Analysis|
Indication |
Placebo Patients |
Drug Patients |
Relative Risk: |
Risk Difference: |
|
Epilepsy |
1.0 |
3.4 |
3.5 |
2.4 |
|
Psychiatric |
5.7 |
8.5 |
1.5 |
2.9 |
|
Other |
1.0 |
1.8 |
1.9 |
0.9 |
|
Total |
2.4 |
4.3 |
1.8 |
1.9 |
The relative risk for suicidal thoughts or behavior was higher in clinical trials for epilepsy than in clinical trials for psychiatric or other conditions, but the absolute risk differences were similar for the epilepsy and psychiatric indications.
Anyone considering prescribing ZONISADE or any other AED must balance the risk of suicidal thoughts or behavior with the risk of untreated illness. Epilepsy and many other illnesses for which AEDs are prescribed are themselves associated with morbidity and mortality and an increased risk of suicidal thoughts and behavior. Should suicidal thoughts and behavior emerge during treatment, the prescriber needs to consider whether the emergence of these symptoms in any given patient may be related to the illness being treated.
5.8 Metabolic Acidosis
Zonisamide causes hyperchloremic, non-anion gap, metabolic acidosis (i.e., decreased serum bicarbonate below the normal reference range in the absence of chronic respiratory alkalosis). This metabolic acidosis is caused by renal bicarbonate loss due to the inhibitory effect of zonisamide on carbonic anhydrase. Generally, zonisamide-induced metabolic acidosis occurs early in treatment, but it can develop at any time during treatment. Metabolic acidosis generally appears dose-dependent and can occur at doses as low as 25 mg daily.
Conditions or therapies that predispose to acidosis (such as renal disease, severe respiratory disorders, status epilepticus, diarrhea, ketogenic diet, or specific drugs) may be additive to the bicarbonate lowering effects of zonisamide.
Some manifestations of acute or chronic metabolic acidosis include hyperventilation, nonspecific symptoms such as fatigue and anorexia, or more severe sequelae including cardiac arrhythmias or stupor. Chronic, untreated, metabolic acidosis may increase the risk for nephrolithiasis or nephrocalcinosis. Nephrolithiasis has been observed in the clinical development program in 4% of adults treated with zonisamide, has also been detected by renal ultrasound in 8% of pediatric treated patients who had at least one ultrasound prospectively collected, and was reported as an adverse event in 3% (4/133) of pediatric patients [see Warnings and Precautions (5.15)]. Metabolic acidosis can also increase the risk for hyperammonemia, particularly in the presence of drugs which can cause hyperammonemia.
Chronic, untreated metabolic acidosis may result in osteomalacia (referred to as rickets in pediatric patients) and/or osteoporosis with an increased risk for fracture. Of potential relevance, zonisamide treatment was associated with reductions in serum phosphorus and increases in serum alkaline phosphatase, changes that may be related to metabolic acidosis and osteomalacia.
Chronic, untreated metabolic acidosis in pediatric patients may reduce growth rates. A reduction in growth rate may eventually decrease the maximal height achieved. The effect of zonisamide on growth and bone-related sequelae has not been systematically investigated. ZONISADE is not approved for use in patients below 16 years of age.
Serum bicarbonate was not measured in the adjunctive controlled trials of adults with epilepsy. However, serum bicarbonate was studied in three clinical trials for indications which have not been approved: a placebo-controlled trial for migraine prophylaxis in adults, a controlled trial for monotherapy in epilepsy in adults, and an open label trial for adjunctive treatment of epilepsy in pediatric patients (3-16 years). In adults, mean serum bicarbonate reductions ranged from approximately 2 mEq/L at daily doses of 100 mg to nearly 4 mEq/L at daily doses of 300 mg. In pediatric patients, mean serum bicarbonate reductions ranged from approximately 2 mEq/L at daily doses from above 100 mg up to 300 mg, to nearly 4 mEq/L at daily doses from above 400 mg up to 600 mg.
In two controlled studies in adults, the incidence of a persistent treatment- emergent decrease in serum bicarbonate to less than 20 mEq/L (observed at 2 or more consecutive visits or the final visit) was dose-related at relatively low zonisamide doses. In the monotherapy trial of epilepsy, the incidence of a persistent treatment-emergent decrease in serum bicarbonate was 21% for daily zonisamide doses of 25 mg or 100 mg, and was 43% at a daily dose of 300 mg. In a placebo-controlled trial for prophylaxis of migraine, the incidence of a persistent treatment-emergent decrease in serum bicarbonate was 7% for placebo, 29% for 150 mg daily, and 34% for 300 mg daily. The incidence of persistent markedly abnormally low serum bicarbonate (decrease to less than 17 mEq/L and more than 5 mEq/L from a pretreatment value of at least 20 mEq/L) in these controlled trials was 2% or less.
In the pediatric study, the incidence of persistent, treatment-emergent decreases in serum bicarbonate to levels less than 20 mEq/L was 52% at doses up to 100 mg daily, was 90% for a wide range of doses up to 600 mg daily, and generally appeared to increase with higher doses. The incidence of a persistent markedly abnormally low serum bicarbonate value was 4% at doses up to 100 mg daily, was 18% for a wide range of doses up to 600 mg daily, and generally appeared to increase with higher doses. Some patients experienced moderately severe serum bicarbonate decrements down to a level as low as 10 mEq/L.
The relatively high frequencies of varying severities of metabolic acidosis observed in this study of pediatric patients (compared to the frequency and severity observed in various clinical trial development programs in adults) suggest that pediatric patients may be more likely to develop metabolic acidosis than adults.
Measurement of baseline and periodic serum bicarbonate during treatment is recommended. If metabolic acidosis develops and persists, consideration should be given to reducing the dose or discontinuing ZONISADE (using dose tapering). If the decision is made to continue patients on ZONISADE in the face of persistent acidosis, alkali treatment should be considered.
5.9 Seizures on Withdrawal of Antiepileptic Drugs
As with most antiepileptic drugs, ZONISADE should generally be withdrawn gradually because of the risk of increased seizure frequency and status epilepticus [see Dosage and Administration (2.3)]. However, if withdrawal is needed because of a serious adverse event, rapid discontinuation can be considered. In these situations, appropriate monitoring is recommended.
5.10 Teratogenicity
Women of childbearing potential who are given ZONISADE should be advised to use effective contraception. Zonisamide produced fetal malformations in mice, rats, and dogs and was embryolethal in monkeys when administered during the period of organogenesis. A variety of fetal abnormalities, including cardiovascular defects and embryofetal deaths, occurred at maternal plasma levels similar to or lower than therapeutic levels in humans. These findings suggest that the use of zonisamide during pregnancy in humans may present a significant risk to the fetus [see Use in Specific Populations (8.1)].
Although human data to confirm findings in animals is limited, ZONISADE should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
5.11 Cognitive/Neuropsychiatric Adverse Reactions
Use of zonisamide was frequently associated with central nervous system- related adverse reactions [see Adverse Reactions (6.1)]. The most significant of these can be classified into three general categories: 1) psychiatric symptoms, including depression and psychosis, 2) cognitive dysfunction, and 3) somnolence or fatigue.
Psychiatric Symptoms
In placebo-controlled trials, 2.2% of patients discontinued zonisamide or were hospitalized for depression compared to 0.4% of placebo patients. Among all epilepsy patients treated with zonisamide, 1.4% were discontinued and 1.0% were hospitalized because of reported depression or suicide attempts. In placebo-controlled trials, 2.2% of patients discontinued zonisamide or were hospitalized because of psychosis or psychosis-related symptoms compared to no patients who received placebo. Among all epilepsy patients treated with zonisamide, 0.9% discontinued treatment and 1.4% were hospitalized because of reported psychosis or related symptoms.
Cognitive Dysfunction
Zonisamide, the active ingredient in ZONISADE, causes adverse reactions related to cognitive dysfunction (e.g., psychomotor slowing, difficulty with concentration, and speech or language problems, in particular, word-finding difficulties). In placebo-controlled trials with zonisamide, psychomotor slowing and difficulty with concentration occurred in the first month of treatment and were associated with doses above 300 mg/day. Speech and language problems tended to occur after 6–10 weeks of treatment and at doses above 300 mg/day. Although in most cases these events were of mild to moderate severity, they at times led to withdrawal from treatment.
Somnolence and Fatigue
Somnolence and fatigue were frequently reported CNS adverse events during clinical trials with zonisamide. Although in most cases these events were of mild to moderate severity, they led to withdrawal from treatment in 0.2% of the patients enrolled in controlled trials. Somnolence and fatigue tended to occur within the first month of treatment. Somnolence and fatigue occurred most frequently at doses of 300–500 mg/day.
Risk Amelioration
Prescribers should advise patients against engaging in hazardous activities requiring mental alertness, such as operating motor vehicles or dangerous machinery, until the effect of ZONISADE is known. Patients should be carefully observed for signs of central nervous system (CNS) depression, such as somnolence and sedation, when ZONISADE is used with other drugs with sedative properties because of potential additive effects.
5.12 Hyperammonemia and Encephalopathy
Hyperammonemia and encephalopathy have been reported with the postmarketing use of zonisamide. Zonisamide, the active ingredient in ZONISADE, treatment inhibits carbonic anhydrase activity, which may cause metabolic acidosis that is associated with an increased risk for developing hyperammonemia. Hyperammonemia resulting from zonisamide can also be asymptomatic.
The risks of hyperammonemia and various manifestations of encephalopathy may be increased in patients treated with zonisamide and concomitantly taking other medications that can cause hyperammonemia, including valproic acid or topiramate [see Warnings and Precautions (5)]. Patients with inborn errors of metabolism or reduced hepatic mitochondrial activity may be at an increased risk for hyperammonemia with or without encephalopathy and this risk may be increased by zonisamide use.
Measure serum ammonia concentration if signs or symptoms (e.g., unexplained change in mental status, vomiting, or lethargy) of encephalopathy occur. Hyperammonemia resulting from zonisamide resolves when zonisamide is discontinued. Hyperammonemia from zonisamide may resolve or decrease in severity with a decrease of the daily dose.
5.13 Kidney Stones
Zonisamide, the active ingredient in ZONISADE, may cause kidney stones. Among
991 patients treated during the development of zonisamide, 40 patients (4.0%)
with epilepsy receiving zonisamide developed clinically possible or confirmed
kidney stones (e.g., clinical symptomatology, sonography, etc.), at rate of 34
per 1000 patient-years of exposure (40 patients with 1168 years of exposure).
Of these, 12 were symptomatic, and 28 were described as possible kidney stones
based on sonographic detection. In nine patients, the diagnosis was confirmed
by a passage of a stone or by a definitive sonographic finding. The rate of
occurrence of kidney stones was 28.7 per 1000 patient-years of exposure in the
first six months, 62.6 per 1000 patient-years of exposure between 6 and 12
months, and 24.3 per 1000 patient-years of exposure after 12 months of use.
There are no normative sonographic data available for either the general
population or patients with epilepsy. Although the clinical significance of
the sonographic findings may not be certain, the development of
nephrolithiasis may be related to metabolic acidosis [see Warnings and Precautions (5.8)]. The analyzed stones were composed of calcium or urate
salts. In general, increasing fluid intake and urine output can help reduce
the risk of stone formation, particularly in those with predisposing risk
factors. It is unknown, however, whether these measures will reduce the risk
of stone formation in patients treated with ZONISADE.
Although not approved in pediatric patients, sonographic findings consistent
with nephrolithiasis were also detected in 8% of a subset of zonisamide
treated pediatric patients who had at least one renal ultrasound prospectively
performed in a clinical development program investigating open-label
treatment. The incidence of kidney stone as an adverse event was 3% [see Warnings and Precautions (5.8)].
5.14 Effect on Renal Function
Zonisamide, the active ingredient in ZONISADE, can have an effect on renal function. In several clinical studies, zonisamide was associated with a statistically significant 8% mean increase from baseline of serum creatinine and blood urea nitrogen (BUN) compared to essentially no change in the placebo patients. The increase appeared to persist over time but was not progressive; this has been interpreted as an effect on glomerular filtration rate (GFR). There were no episodes of unexplained acute renal failure in clinical development in the US, Europe, or Japan. The decrease in GFR appeared within the first 4 weeks of treatment. In a 30-day study, the GFR returned to baseline within 2–3 weeks of drug discontinuation. There is no information about reversibility, after drug discontinuation, of the effects on GFR after long-term use. ZONISADE should be discontinued in patients who develop acute renal failure or a clinically significant sustained increase in the creatinine/BUN concentration. Avoid use of ZONISADE in patients with renal failure (estimated GFR < 50 mL/min) since there is insufficient experience concerning drug dosing and toxicity [see Use in Specific Populations (8.6)]. Consideration should be given to monitoring renal function periodically.
5.15 Status Epilepticus
Estimates of the incidence of treatment emergent status epilepticus in patients treated with zonisamide, the active ingredient in ZONISADE, are difficult because a standard definition was not employed. Nonetheless, in controlled trials, 1.1% of patients treated with zonisamide had an event labeled as status epilepticus compared to none of the patients treated with placebo. Among patients treated with zonisamide across all epilepsy studies (controlled and uncontrolled), 1.0% of patients had an event reported as status epilepticus.
•
Potentially Fatal Reactions to Sulfonamides: Fatalities have occurred as a result of severe reactions to sulfonamides (zonisamide is a sulfonamide) including Stevens-Johnson syndrome, toxic epidermal necrolysis, fulminant hepatic necrosis, agranulocytosis, aplastic anemia, and other blood dyscrasias (5.1[).](http://file///Y:/Supp/Gaurav/Zonisade-SPL/Working/pi-word-seq0025.docx)
•
Serious Skin Reactions: Discontinue ZONISADE at the first sign of rash unless clearly not drug related (5.2).
•
Serious Hematologic Events: Aplastic anemia and agranulocytosis have been reported with zonisamide treatment (5.3).
•
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multi-Organ Hypersensitivity: DRESS, also known as multiorgan hypersensitivity, has occurred with zonisamide (5.4).
•
Oligohidrosis and Hyperthermia in Pediatric Patients: Oligohidrosis, sometimes resulting in heat stroke and hospitalization, is seen in association with zonisamide in pediatric patients (5.5).
•
Acute Myopia and Secondary Angle Closure Glaucoma: If occurs, primary treatment is discontinuation of ZONISADE (5.6).
•
Suicidal Behavior and Ideation: Monitor patients for suicidal behavior or ideation (5.7).
•
Metabolic Acidosis: Baseline and periodic measurement of serum bicarbonate is recommended; consider dose reduction or discontinuation if appropriate (5.8).
•
Seizures on Withdrawal of Antiepileptic Drugs: Withdraw ZONISADE gradually (5.9).
•
Teratogenicity: Based on animal data, may cause fetal harm. Advise females of reproductive potential of the potential risk to a fetus and to use an effective method of contraception during ZONISADE treatment and for one month after discontinuation (5.10, 8.1, 8.3).
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 CNS Depressants
Concomitant use of ZONISADE with other CNS depressants, including alcohol, may increase the risk of CNS depression, as well as other cognitive and/or neuropsychiatric adverse events [see Warnings and Precautions (5.11)].
7.2 Other Carbonic Anhydrase Inhibitors
Concomitant use of ZONISADE, a carbonic anhydrase inhibitor, with any other carbonic anhydrase inhibitor, may increase the severity of metabolic acidosis and may also increase the risk of kidney stone formation [see Warnings and Precautions (5.8, 5.15)]. Therefore, if ZONISADE is given concomitantly with another carbonic anhydrase inhibitor, monitor the patient for the appearance or worsening of metabolic acidosis [see Clinical Pharmacology (12.1, 12.3)].
7.3 CYP3A4 Inducers
If co-administration with a potent CYP3A4 inducer is necessary, the patient should be closely monitored and the dose of ZONISAMIDE and other drugs that CYP3A4 substrates may need to be adjusted [see Clinical Pharmacology (12.3)].
•
ZONISADE should be used with caution if used in combination with alcohol or other CNS depressants (7.1).
•
Concomitant use of ZONISADE with any other carbonic anhydrase inhibitor may increase the severity of metabolic acidosis and may also increase the risk of kidney stone formation (7.2).
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Assessments for Safety
To assess for metabolic acidosis, obtain baseline serum bicarbonate prior to initiating ZONISADE, and obtain periodic serum bicarbonate during treatment [see Warnings and Precautions (5.8)].
2.2 Recommended Dosage
Administer ZONISADE once or twice daily with or without food.
The recommended initial dosage of ZONISADE is 100 mg daily. The dosage may be increased by 100 mg daily every two weeks, based on clinical response and tolerability, to 400 mg daily. Patients who are tolerating ZONISADE at 400 mg daily and require further reduction of seizures may be increased up to a maximum dosage of 600 mg daily. However, evidence from controlled trials shows no suggestion of increasing response above 400 mg/day [see Clinical Studies (14)].
2.3 Important Administration Information
Shake well before every administration. To administer ZONISADE directly into the mouth, it is important that ZONISADE be measured with an accurate measuring device [see Overdosage (10)]. A household teaspoon is not an accurate measuring device. A pharmacist will provide an appropriate device and instructions for measuring the correct dose.
Administer ZONISADE orally with or without food.
Discard unused portion of ZONISADE 30 days after first opening the bottle.
2.4 Discontinuation of ZONISADE
When discontinuing ZONISADE, the dose should be decreased gradually. As with most antiepileptic drugs, avoid abrupt discontinuation, when possible, to minimize the risk of increased seizure frequency and status epilepticus [see Warnings and Precautions (5.9)].
•
The recommended initial dosage of ZONISADE is 100 mg daily. The dosage may be increased by 100 mg daily every two weeks, based on clinical response and tolerability, to 400 mg daily. Patients who are tolerating ZONISADE at 400 mg daily and require further reduction of seizures may be increased up to a maximum dosage of 600 mg daily (2.2).
•
ZONISADE is given orally and can be taken with or without food (2.2).
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The precise mechanism(s) by which zonisamide exerts its anticonvulsant effects is unknown. Zonisamide may produce these effects through action at sodium and calcium channels. In vitro pharmacological studies suggest that zonisamide blocks sodium channels and reduces voltage-dependent, transient inward currents (T-type Ca2+ currents), consequently stabilizing neuronal membranes. Other in vitro studies have demonstrated that zonisamide (10–30 µg/mL) suppresses synaptically-driven electrical activity without affecting postsynaptic GABA or glutamate responses (cultured mouse spinal cord neurons) or neuronal or glial uptake of [3H]-GABA (rat hippocampal slices). Thus, zonisamide does not appear to potentiate the synaptic activity of GABA. Zonisamide is a carbonic anhydrase inhibitor. The contribution of this pharmacological action to the therapeutic effects of zonisamide is unknown.
12.2 Pharmacodynamics
As a carbonic anhydrase inhibitor, ZONISADE may cause metabolic acidosis and may also increase the risks of hyperammonemia and kidney stone formation [see Warnings and Precautions (5.8, 5.13, 5.15) and Drug Interactions (7.2)].
12.3 Pharmacokinetics
Absorption
Following a 100 mg ZONISADE dose in normal volunteers, the time to maximum plasma concentrations (Tmax) occurred within 0.5–5 hours.
Zonisamide pharmacokinetics are dose-proportional in the range of 200 to 400 mg. Once a stable dose is reached, steady state is achieved within 14 days.
Effect of Food
When ZONISADE is administered with food, the zonisamide Tmax is delayed, occurring at 3.5–7.5 hours, but food has no effect on the bioavailability of zonisamide.
Distribution
The apparent volume of distribution (V/F) of zonisamide is about 1.45 L/kg following a 400 mg oral dose. Zonisamide, at concentrations of 1.0–7.0 mcg/mL, is approximately 40% bound to human plasma proteins. Zonisamide extensively binds to erythrocytes, resulting in an eight-fold higher concentration of zonisamide in red blood cells than in plasma. Protein binding of zonisamide is unaffected in the presence of therapeutic concentrations of phenytoin, phenobarbital, or carbamazepine.
Elimination
The plasma clearance of oral zonisamide is approximately 0.30–0.35 mL/min/kg in patients not receiving enzyme-inducing antiepileptic drugs (AEDs). The clearance of zonisamide is increased to 0.5 mL/min/kg in patients concurrently on enzyme-inducing AEDs (see Potential for Other Drugs to Affect ZONISADE). After a single-dose administration, renal clearance of zonisamide is approximately 3.5 mL/min.
Metabolism
Zonisamide is metabolized by N-acetyl-transferases to form N-acetyl zonisamide and by CYP3A4 to form 2–sulfamoylacetylphenol (SMAP).
Excretion
The elimination half-life of zonisamide in plasma is approximately 63 hours. The elimination half-life of zonisamide in red blood cells is approximately 105 hours. Zonisamide is excreted primarily in urine as parent drug and as the glucuronide of a metabolite. Following multiple dosing, 62% of the radiolabeled dose was recovered in the urine, with 3% in the feces by day 10. Of the excreted dose, 35% was recovered as zonisamide, 15% as N-acetyl zonisamide, and 50% as the glucuronide of SMAP.
Specific Populations
Patients with Renal Impairment
Single 300 mg zonisamide doses were administered to three groups of volunteers. Group 1 was a healthy group with a creatinine clearance ranging from 70–152 mL/min. Group 2 and Group 3 had creatinine clearances ranging from 14.5–59 mL/min and 10–20 mL/min, respectively. Zonisamide renal clearance decreased with decreasing renal function (3.42, 2.50, and 2.23 mL/min, respectively). Marked renal impairment (creatinine clearance < 20 mL/min) was associated with an increase in zonisamide AUC of 35% [see Use in Specific Populations (8.6)].
Patients with Hepatic Impairment
The pharmacokinetics of zonisamide in patients with impaired liver function have not been studied.
Age
The pharmacokinetics of a 300 mg single dose of zonisamide were similar in young (mean age 28 years) and elderly subjects (mean age 69 years).
Drug Interaction Studies
In-Vitro Studies
Enzymes
In vitro studies using human liver microsomes show insignificant (<25%) inhibition of cytochrome P450 isozymes 1A2, 2A6, 2C9, 2C19, 2D6, 2E1, 3A4, 2B6, or 2C8 at zonisamide levels approximately two-fold or greater than clinically relevant unbound serum concentrations. Therefore, ZONISADE is not expected to affect the pharmacokinetics of other drugs via cytochrome P450-mediated mechanisms.
Transporters
An in-vitro study showed that zonisamide is a weak inhibitor of P-gp (MDR1).
In-Vivo Studies
Potential for Zonisamide to Affect Other Drugs
Antiepileptic Drugs
In epileptic patients, steady state dosing with zonisamide capsules resulted in no clinically relevant pharmacokinetic effects on carbamazepine, lamotrigine, phenytoin, or sodium valproate.
Oral Contraceptives
In healthy subjects, steady state dosing with zonisamide capsules did not affect serum concentrations of ethinylestradiol or norethisterone in a combined oral contraceptive.
CYP2D6 Substrates
Coadministration of multiple dosing of zonisamide up to 400 mg/day with single 50-mg doses of desipramine did not significantly affect the pharmacokinetic parameters of desipramine, a probe drug for CYP2D6 activity.
Potential for Other Drugs to Affect ZONISADE
CYP3A4 Inducers
The half-life of zonisamide following a 400 mg dose in patients concurrently on enzyme-inducing AEDs such as phenytoin, carbamazepine, or phenobarbital, was between 27-38 hours; the half-life of zonisamide in patients concurrently on the non-enzyme inducing AED, valproate, was 46 hours.
These effects are unlikely to be of clinical significance when ZONISADE is added to existing therapy; however, changes in zonisamide concentrations may occur if concomitant CYP3A4 inducing antiepileptic or other drugs are withdrawn, dose adjusted or introduced, an adjustment of the ZONISADE dose may be required [see Drug Interactions (7.3)].
CYP3A4 Inhibitors
Steady-state dosing of either ketoconazole (400 mg/day) or cimetidine (1200 mg/day) had no clinically relevant effects on the single dose pharmacokinetics of zonisamide given to healthy subjects.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenicity, Mutagenesis, Impairment of Fertility
Carcinogenicity
No evidence of carcinogenicity was found in mice or rats following dietary administration of zonisamide for two years at doses of up to 80 mg/kg/day. In mice, this dose is approximately equivalent to the maximum recommended human dose (MRHD) of 400 mg/day on a mg/m2 basis. In rats, this dose is 1–2 times the MRHD on a mg/m2 basis.
Mutagenesis
Zonisamide was mutagenic in an in vitro chromosomal aberration assay in CHL cells. Zonisamide was not mutagenic or clastogenic in other in vitro assays (Ames, mouse lymphoma tk assay, chromosomal aberration in human lymphocytes) or in the in vivo rat bone marrow cytogenetics assay.
Impairment of Fertility
Rats treated with zonisamide (20, 60, or 200 mg/kg) before mating and during the initial gestation phase showed signs of reproductive toxicity (decreased corpora lutea, implantations, and live fetuses) at all doses. The low dose in this study is approximately 0.5 times the maximum recommended human dose (MRHD) on a mg/m2 basis.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
The efficacy of ZONISADE is based upon a bioavailability study comparing ZONISADE oral suspension to zonisamide capsules in healthy subjects. The clinical studies information described below pertains to the zonisamide capsule formulation.
The effectiveness of zonisamide as adjunctive therapy has been established in three multicenter, placebo-controlled, double blind, 3-month clinical trials (two domestic, one European) in 499 patients with refractory partial-onset seizures with or without secondary generalization. Each patient had a history of at least four partial-onset seizures per month in spite of receiving one or two antiepilepsy drugs at therapeutic concentrations. The 499 patients (209 women, 290 men) had a mean age of about 35 years. In the two US studies, over 80% of patients were Caucasian; 100% of patients in the European study were Caucasian. Zonisamide capsules or placebo was added to the existing therapy. The primary measure of effectiveness was median percent reduction from baseline in partial seizure frequency. The secondary measure was proportion of patients achieving a 50% or greater seizure reduction from baseline (responders). The results described below are for all partial seizures in the intent-to-treat populations.
In the first study (n = 203), all patients had a 1-month baseline observation period, then received placebo or zonisamide capsules in one of two dose escalation regimens; either 1) 100 mg/day for five weeks, 200 mg/day for one week, 300 mg/day for one week, and then 400 mg/day for five weeks; or 2) 100 mg/day for one week, followed by 200 mg/day for five weeks, then 300 mg/day for one week, then 400 mg/day for five weeks. This design allowed a 100 mg vs. placebo comparison over weeks 1–5, and a 200 mg vs. placebo comparison over weeks 2–6; the primary comparison was 400 mg (both escalation groups combined) vs. placebo over weeks 8–12. The total daily dose was given as twice a day dosing. Statistically significant treatment differences favoring zonisamide were seen for doses of 100, 200, and 400 mg/day.
In the second (n = 152) and third (n = 138) studies, patients had a 2–3 month baseline, then were randomly assigned to placebo or zonisamide capsules for three months. Zonisamide was introduced by administering 100 mg/day for the first week, 200 mg/day the second week, then 400 mg/day for two weeks, after which the dose could be adjusted as necessary to a maximum dose of 20 mg/kg/day or a maximum plasma level of 40 µg/mL. In the second study, the total daily dose was given as twice a day dosing; in the third study, it was given as a single daily dose. The average final maintenance doses received in the studies were 530 and 430 mg/day in the second and third studies, respectively. Both studies demonstrated statistically significant differences favoring zonisamide for doses of 400–600 mg/day, and there was no apparent difference between once daily and twice daily dosing (in different studies). Analysis of the data (first 4 weeks) during titration demonstrated statistically significant differences favoring zonisamide at doses between 100 and 400 mg/day. The primary comparison in both trials was for any dose over Weeks 5–12.
Table 3. Median % Reduction in All Partial-Onset Seizures and % Responders in Primary Efficacy Analyses: Intent-To-Treat Analysis
| ||||
|
Study |
Median % Reduction |
% Responders | ||
|
Zonisamide Capsules |
Placebo |
Zonisamide Capsules |
Placebo | |
|
Study 1: |
n=98 |
n=72 |
n=98 |
n=72 |
|
Weeks 8-12: |
40.5%* |
9.0% |
41.8%* |
22.2% |
|
Study 2: |
n=69 |
n=72 |
n=69 |
n=72 |
|
Weeks 5-12: |
29.6%* |
-3.2% |
29.0% |
15.0% |
|
Study 3: |
n=67 |
n=66 |
n=67 |
n=66 |
|
Weeks 5-12: |
27.2%* |
-1.1% |
28.0%* |
12.0% |
| ||||
|
Dose Group |
Median % Reduction |
% Responders | ||
|
Zonisamide Capsules |
Placebo |
Zonisamide Capsules |
Placebo | |
|
100-400 mg/day: |
n=112 |
n=83 |
n=112 |
n=83 |
|
Weeks 1-12: |
32.3%* |
5.6% |
32.1%* |
9.6% |
|
100 mg/day: |
n=56 |
n=80 |
n=56 |
n=80 |
|
Weeks 1-5: |
24.7%* |
8.3% |
25.0%* |
11.3% |
|
200 mg/day: |
n=55 |
n=82 |
n=55 |
n=82 |
|
Weeks 2-6: |
20.4%* |
4.0% |
25.5%* |
9.8% |
In Figure 1, a positive value on the Y-axis indicates an improvement from baseline (i.e., a decrease in seizure rate), while a negative value indicates a worsening from baseline (i.e., an increase in seizure rate). Thus, in a display of this type, the curve for an effective treatment is shifted to the left of the curve for placebo. The proportion of patients achieving any particular level of reduction in seizure rate was consistently higher for the zonisamide groups compared to the placebo groups. For example, Figure 1 indicates that approximately 27% of patients treated with zonisamide experienced a 75% or greater reduction, compared to approximately 12% in the placebo groups.
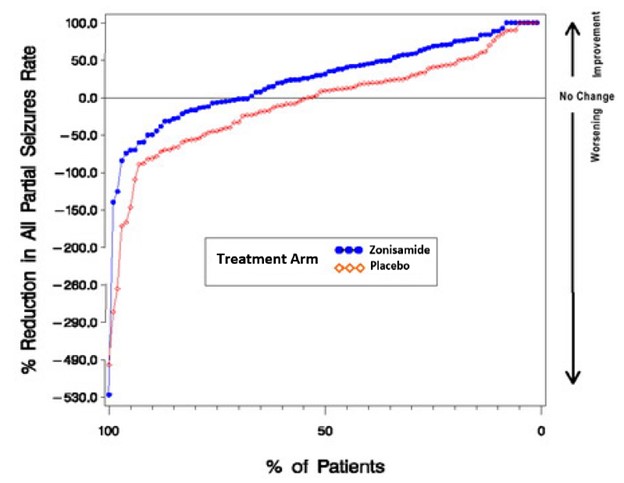
Figure 1. Proportion of Patients Achieving Differing Levels of Seizure Reduction in Zonisamide and Placebo Groups in Studies 2 and 3
No differences in efficacy based on age, sex or race, as measured by a change in seizure frequency from baseline, were detected.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
ZONISADE (zonisamide oral suspension) is a white to off-white, strawberry flavored liquid containing 100 mg/5 mL zonisamide. It is supplied in a 150 mL amber colored PET bottle with a child resistant cap.
NDC Number: 52652-8001-1
16.2 Storage and Handling
Store at 20°C to 25°C (68°F to 77°F), excursions permitted from 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]. Protect from light.
Discard unused portion of ZONISADE 30 days after first opening of the bottle.
