HEALSURE PAIN RELIEF
84010-124
36066488-0bf3-05c7-e063-6394a90a3baa
HUMAN OTC DRUG LABEL
May 26, 2025
Jiangxi Hemei Pharmaceutical Co., Ltd
DUNS: 724892056
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Arnica montana HPUS 7% PAIN RELIEF
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
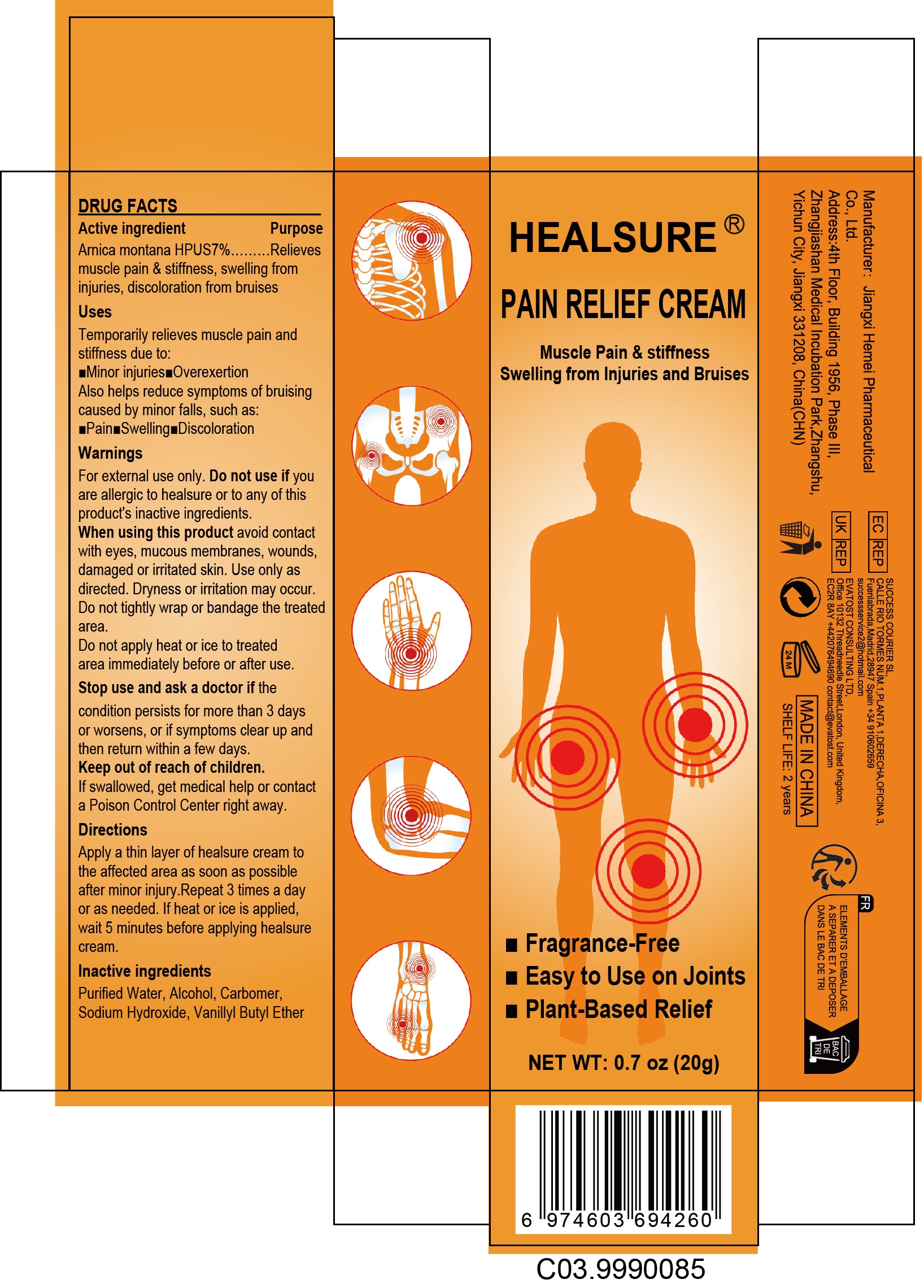
INDICATIONS & USAGE SECTION
Use
Temporarily relieves muscle pain and stiffness due to:
■Minor injuries■Overexertio
Also helps reduces symptoms of bruising such as:
■Pain■Swelling■Discoloration
OTC - ACTIVE INGREDIENT SECTION
Active Ingredient
Arnica montana HPUS7%
OTC - PURPOSE SECTION
Purpose
Relieves muscle pain & stiffness,swelling from injuries, discoloration from bruises
WARNINGS SECTION
Warnings
For external use only
OTC - DO NOT USE SECTION
Do not use
Do not use if you are allergic to healsure or to any of this product's inactive ingredients
OTC - WHEN USING SECTION
When Using
When using this product avoid contact with eyes, mucous membranes, wounds
damaged or irritated skin.Use only as directed. Dryness or irritation may
occur
Do not tightly wrap or bandage the treated area. Do not apply heat or ice to
treated area immediately before or after use
OTC - STOP USE SECTION
Stop Use
if teh ocondition persists for more than 3 days or worsensosor if symptoms clear up and then retum within a few days.
OTC - ASK DOCTOR SECTION
Ask Doctor
if teh ocondition persists for more than 3 days or worsensosor if symptoms clear up and then retum within a few days.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep Out Of Reach Of Children
lf swallowed, get medical help or contact a Poison Control Center right away
DOSAGE & ADMINISTRATION SECTION
Directions
Apply a thin layer of dermfree cream to the affected area as soon as possible after minor injury. Repeat 3 times a day or asneeded.lf heat or ice is applied, wait 5 minutes before applying dermfree cream.
INACTIVE INGREDIENT SECTION
Inactive ingredients
Purifed Water, Alcohol, Carbomer, Sodium Hydroxide, Vanillyl Butyl Ether
