Acetylcysteine
Acetylcysteine Solution, USP
c90fca12-12fb-4cf6-bd6f-83bc0d95df81
HUMAN PRESCRIPTION DRUG LABEL
Mar 20, 2024
Cardinal Health 107, LLC
DUNS: 118546603
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Acetylcysteine
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (4)
Acetylcysteine
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (4)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Package/Label Display Panel
Acetylcysteine Solution, USP
20% (200 mg/ml)
5 x 4 ml Vials

INDICATIONS & USAGE SECTION
INDICATIONS AND USAGE
Acetylcysteine, administered orally, is indicated as an antidote to prevent or lessen hepatic injury which may occur following the ingestion of a potentially hepatotoxic quantity of acetaminophen.
It is essential to initiate treatment as soon as possible after the overdose and, in any case, within 24 hours of ingestion.
CONTRAINDICATIONS SECTION
CONTRAINDICATIONS
There are no contraindications to oral administration of acetylcysteine in the treatment of acetaminophen overdose.
ADVERSE REACTIONS SECTION
ADVERSE REACTIONS
Oral administration of acetylcysteine, especially in the large doses needed to treat acetaminophen overdose, may result in nausea, vomiting and other gastrointestinal symptoms. Rash with or without mild fever has been observed rarely.
DESCRIPTION SECTION
DESCRIPTION
Acetylcysteine is for inhalation (mucolytic agent) or oral administration (acetaminophen antidote), available as a sterile, unpreserved solution (NOT FOR INJECTION). The solutions contain 20% (200 mg/mL) or 10% (100 mg/mL) acetylcysteine, with disodium edetate in water for injection. Sodium hydroxide and/or hydrochloric acid is added to adjust pH (range 6.0 - 7.5). Acetylcysteine is the N-acetyl derivative of the naturally-occurring amino acid, L-cysteine. The compound is a white crystalline powder with the molecular formula C5H9NO3S, a molecular weight of 163.2, and chemical name of N-acetyl-L-cysteine. Acetylcysteine has the following structural formula:

This product contains the following inactive ingredients:
disodium edetate, sodium hydroxide and water for injection.
CLINICAL PHARMACOLOGY SECTION
CLINICAL PHARMACOLOGY
(Antidotal) Acetaminophen is rapidly absorbed from the upper gastrointestinal tract with peak plasma levels occurring between 30 and 60 minutes after therapeutic doses and usually within 4 hours following an overdose. The parent compound, which is nontoxic, is extensively metabolized in the liver to form principally the sulfate and glucuronide conjugates which are also nontoxic and are rapidly excreted in the urine. A small fraction of an ingested dose is metabolized in the liver by the cytochrome P-450 mixed function oxidase enzyme system to form a reactive, potentially toxic, intermediate metabolite which preferentially conjugates with hepatic glutathione to form the nontoxic cysteine and mercapturic acid derivatives which are then excreted by the kidney. Therapeutic doses of acetaminophen do not saturate the glucuronide and sulfate conjugation pathways and do not result in the formation of sufficient reactive metabolite to deplete glutathione stores. However, following ingestion of a large overdose (150 mg/kg or greater) the glucuronide and sulfate conjugation pathways are saturated resulting in a larger fraction of the drug being metabolized via the P-450 pathway. The increased formation of reactive metabolite may deplete the hepatic stores of glutathione with subsequent binding of the metabolite to protein molecules within the hepatocyte resulting in cellular necrosis.
Acetylcysteine has been shown to reduce the extent of liver injury following acetaminophen overdose. Its effectiveness depends on early oral administration, with benefit seen principally in patients treated within 16 hours of the overdose. Acetylcysteine probably protects the liver by maintaining or restoring the glutathione levels, or by acting as an alternate substrate for conjugation with, and thus detoxification of, the reactive metabolite.
REFERENCES SECTION
REFERENCES
Bonanomi L, Gazzaniga A. Toxicological, pharmacokinetic and metabolic studies on acetylcysteine. Eur J Respir Dis, 1981; 61 (Suppl III): 45-51.
Am Rev Respir Dis, 1960; 82:627-639.
Rx Only
IN7504
Rev. 5/14
MG #11105
AMERICAN
REGENT, INC.
SHIRLEY, NY 11967
Distributed By:
Cardinal Health
Dublin, OH 43017
L35753540324
L35753390324
WARNINGS SECTION
WARNINGS
Generalized urticaria has been observed rarely in patients receiving oral acetylcysteine for acetaminophen overdose. If this occurs or other allergic symptoms appear, treatment with acetylcysteine should be discontinued unless it is deemed essential and the allergic symptoms can be otherwise controlled.
If encephalopathy due to hepatic failure becomes evident, acetylcysteine treatment should be discontinued to avoid further administration of nitrogenous substances. There are no data indicating that acetylcysteine influences hepatic failure, but this remains a theoretical possibility.
PRECAUTIONS SECTION
PRECAUTIONS
Occasionally severe and persistent vomiting occurs as a symptom of acute acetaminophen overdose. Treatment with oral acetylcysteine may aggravate the vomiting. Patients at risk of gastric hemorrhage (eg, esophageal varices, peptic ulcers, etc.) should be evaluated concerning the risk of upper gastrointestinal hemorrhage versus the risk of developing hepatic toxicity, and treatment with acetylcysteine given accordingly.
Dilution of the acetylcysteine (see Preparation of Acetylcysteine for Oral Administration) minimizes the propensity of oral acetylcysteine to aggravate vomiting.
DOSAGE & ADMINISTRATION SECTION
DOSAGE AND ADMINISTRATION
General
Regardless of the quantity of acetaminophen reported to have been ingested, administer acetylcysteine immediately if 24 hours or less have elapsed from the reported time of ingestion of an overdose of acetaminophen. Do not await results of assays for acetaminophen level before initiating treatment with acetylcysteine. The following procedures are recommended:
The stomach should be emptied promptly by lavage or by inducing emesis with syrup of ipecac. Syrup of ipecac should be given in a dose of 15 mL for children up to age 12 and 30 mL for adolescents and adults followed immediately by drinking copious amounts of water. The dose should be repeated if emesis does not occur in 20 minutes.
In the case of a mixed drug overdose activated charcoal may be indicated. However, if activated charcoal has been administered, lavage before administering acetylcysteine treatment. Activated charcoal adsorbs acetylcysteine in vitro and may do so in patients and thereby may reduce its effectiveness.
Draw blood for predetoxification acetaminophen plasma assay and baseline SGOT, SGPT, bilirubin, prothrombin time, creatinine, BUN, blood sugar and electrolytes.
Administer the loading dose of acetylcysteine, 140 mg per kg of body weight. (Prepare acetylcysteine for oral administration as described in the Dosage Guide and Preparation table).
Determine subsequent action based on predetoxification plasma acetaminophen information. Choose**ONE** of the following four courses of therapy.
A.
Predetoxification plasma acetaminophen level is clearly in the toxic range (See Acetaminophen Assays - Interpretation and Methodology below):
Administer a first maintenance dose (70 mg/kg acetylcysteine) 4 hours after the loading dose. The maintenance dose is then repeated at 4-hour intervals for a total of 17 doses. Monitor hepatic and renal function and electrolytes throughout the detoxification process.
B.
Predetoxification acetaminophen level could not be obtained
Proceed as in A.
C.
Predetoxification acetaminophen level is clearly in the non-toxic range (beneath the dashed line on the nomogram) and you know that acetaminophen overdose occurred at least 4 hours before the predetoxification acetaminophen plasma assays
Discontinue administration of acetylcysteine.
D.
Predetoxification acetaminophen level was in the non-toxic range, but time of ingestion was unknown or less than 4 hours
Because the level of acetaminophen at the time of predetoxification assay may not be a peak value (peak may not be achieved before 4 hours post-ingestion), obtain a second plasma level in order to decide whether or not the full 17-dose detoxification treatment is necessary.
If the patient vomits an oral dose within 1 hour of administration, repeat that dose.
In the occasional instances where the patient is persistently unable to retain the orally administered acetylcysteine, the antidote may be administered by duodenal intubation.
Repeat SGOT, SGPT, bilirubin, prothrombin time, creatinine, BUN, blood sugar and electrolytes daily if the acetaminophen plasma level is in the potentially toxic range as discussed below.
Preparation of Acetylcysteine for Oral Administration
Oral administration requires dilution of the 20% solution with diet cola or other diet soft drinks, to a final concentration of 5% (see Dosage Guide and Preparation table). If administered via gastric tube or Miller-Abbott tube, water may be used as the diluent. The dilutions should be freshly prepared and utilized within one hour. Remaining undiluted solutions in opened vials can be stored in the refrigerator up to 96 hours.
ACETYLCYSTEINE IS NOT APPROVED FOR PARENTERAL INJECTION.
HOW SUPPLIED SECTION
HOW SUPPLIED
Acetylcysteine is available in rubber stoppered glass vials containing 4, 10, or 30 mL. The 20% solution may be diluted to a lesser concentration with either Sodium Chloride for Injection, Sodium Chloride for Inhalation, Sterile Water for Injection, or Sterile Water for Inhalation. The 10% solution may be used undiluted.
Acetylcysteine solution is sterile and can be used for inhalation (mucolytic agent) or oral administration (acetaminophen antidote). Acetylcysteine solution is not for parenteral injection. It is available as:
Acetylcysteine 20% solution (200 mg acetylcysteine per mL). Sterile, not for injection.
NDC 55154-5731-5 Overbagged with five 4 mL vials
Acetylcysteine 10% solution (100 mg acetylcysteine per mL). Sterile, not for injection.
NDC 55154-5730-5 Overbagged with five 4 mL vials
STORAGE AND HANDLING SECTION
STORAGE
Store unopened vials at 20°-25°C (68°-77°F); excursions permitted to 15°-30°C (59°-86°F) (see USP Controlled Room Temperature).
Acetylcysteine solution does not contain an antimicrobial agent, and care must be taken to minimize contamination of the sterile solution. Dilutions of acetylcysteine should be used freshly prepared and utilized within one hour. If only a portion of the solution in a vial is used, store the remaining undiluted portion in a refrigerator and use within 96 hours. A change in color may occur after opening. This does not change the efficacy of the drug.
SPL UNCLASSIFIED SECTION
DOSAGE GUIDE AND PREPARATION
Doses in relation to body weight are:
Loading Dose of Acetylcysteine**|
**If patient weighs less than 20 kg (usually patients younger than 6 years), calculate the dose of acetylcysteine. Each mL of 20% acetylcysteine solution contains 200 mg of acetylcysteine. The loading dose is 140 mg per kilogram of body weight. The maintenance dose is 70 mg/kg. Three (3) mL of diluent are added to each mL of 20% acetylcysteine solution. Do not decrease the proportion of diluent. | |||||
|
grams |
mL of 20% |
mL of |
Total mL of | ||
|
Body Weight |
Acetylcysteine |
Acetylcysteine |
Diluent |
5% Solution | |
|
(kg) |
(lb) | ||||
|
100-109 |
220-240 |
15 |
75 |
225 |
300 |
|
90-99 |
198-218 |
14 |
70 |
210 |
280 |
|
80-89 |
176-196 |
13 |
65 |
195 |
260 |
|
70-79 |
154-174 |
11 |
55 |
165 |
220 |
|
60-69 |
132-152 |
10 |
50 |
150 |
200 |
|
50-59 |
110-130 |
8 |
40 |
120 |
160 |
|
40-49 |
88-108 |
7 |
35 |
105 |
140 |
|
30-39 |
66-86 |
6 |
30 |
90 |
120 |
|
20-29 |
44-64 |
4 |
20 |
60 |
80 |
|
Maintenance Dose****** | |||||
|
(kg) |
(lb) | ||||
|
100-109 |
220-240 |
7.5 |
37 |
113 |
150 |
|
90-99 |
198-218 |
7 |
35 |
105 |
140 |
|
80-89 |
176-196 |
6.5 |
33 |
97 |
130 |
|
70-79 |
154-174 |
5.5 |
28 |
82 |
110 |
|
60-69 |
132-152 |
5 |
25 |
75 |
100 |
|
50-59 |
110-130 |
4 |
20 |
60 |
80 |
|
40-49 |
88-108 |
3.5 |
18 |
52 |
70 |
|
30-39 |
66-86 |
3 |
15 |
45 |
60 |
|
20-29 |
44-64 |
2 |
10 |
30 |
40 |
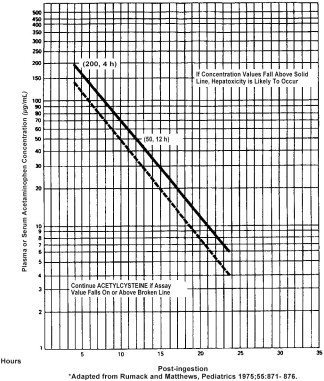
Estimating Potential for Hepatoxicity
The following nomogram has been developed to estimate the probability that plasma levels in relation to intervals post ingestion will result in hepatoxicity.
** Plasma or Serum Acetaminophen Concentration**** v**** Time Post- acetaminophen Ingestion**