Vax-Sode
DRUG FACTS:
cd570e98-c73f-4b38-b5b9-9f16f745ca19
HUMAN OTC DRUG LABEL
Sep 22, 2025
Nutritional Specialties, Inc.
DUNS: 032744609
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Thuja Occidentalis, Calcarea Carbonica, Silicea, Salmonella Typhi Nosode, Serum Anguillae, Bacillus Tetani, Malandrinum, Morbillinum, Parotidinum, Rubella Nosode, Yersinia Enterocolitica
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (13)
Drug Labeling Information
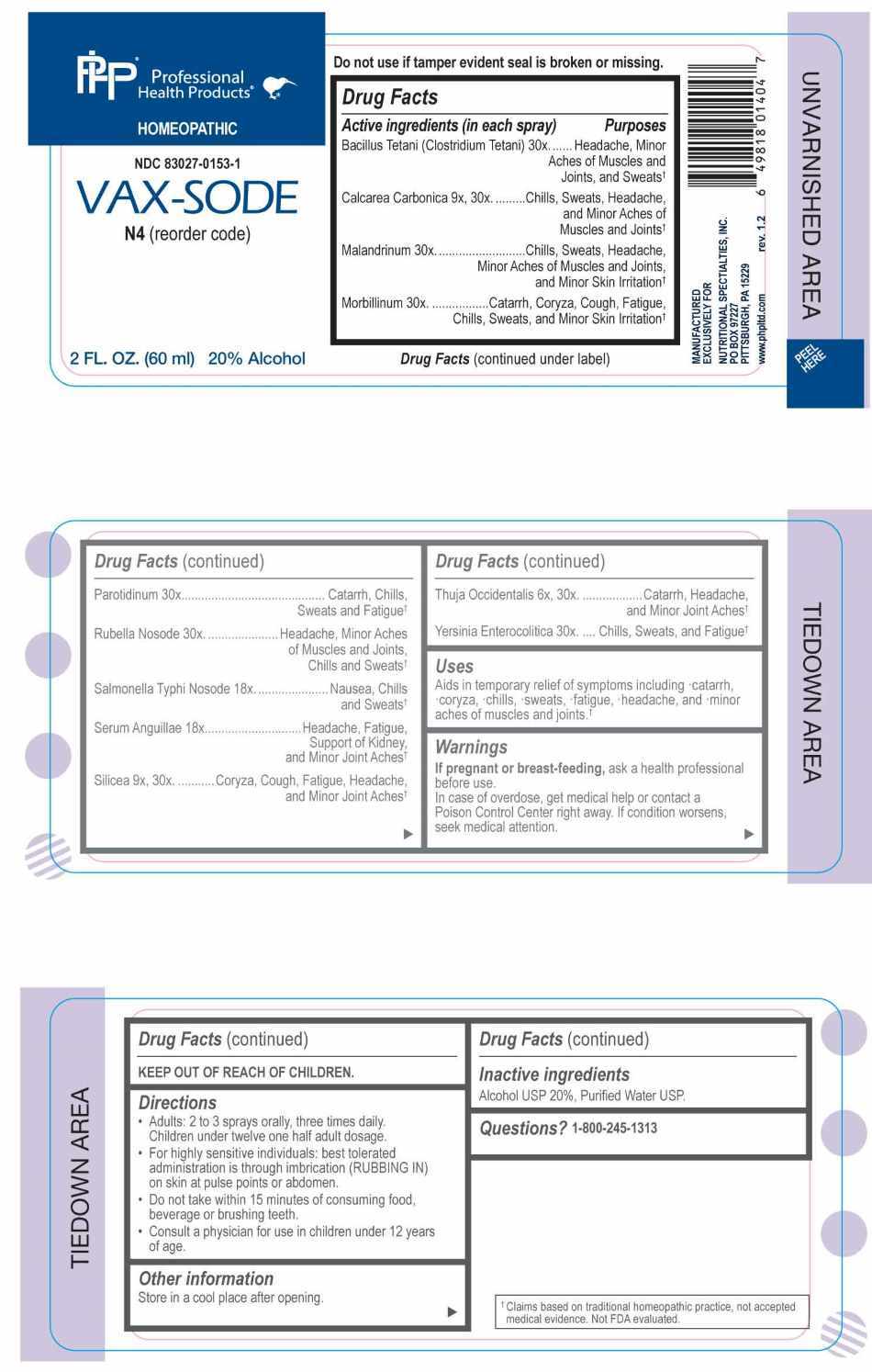
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL DISPLAY:
Professional Health Products
HOMEOPATHIC
NDC 83027-0153-1
VAX-SODE
2 FL. OZ. (60ml)
INDICATIONS & USAGE SECTION
USES:
Aids in temporary relief of symptoms including catarrh, coryza, chills, sweats, fatigue, headache, and minor aches of muscles and joints.†
†Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
OTC - ACTIVE INGREDIENT SECTION
ACTIVE INGREDIENTS:
Bacillus Tetani (Clostridium Tetani) 30X, Calcarea Carbonica 9X, 30X , Malandrinum 30X, Morbillinum 30X, Parotidinum 30X, Rubella Nosode 30X, Salmonella Typhi Nosode 18X, Serum Anguillae 18X, Silicea 9X, 30X, Thuja Occidentalis 6X, 30X, Yersinia Enterocolitica 30X.
OTC - PURPOSE SECTION
PURPOSE:
Bacillus Tetani (Clostridium Tetani) – Headache, Minor Aches of Muscles and Joints, and Sweats,† Calcarea Carbonica – Chills, Sweats, Headache, and Minor Aches of Muscles and Joints,† Malandrinum – Chills, Sweats, Headache, Minor Aches of Muscles and Joints, and Minor Skin Irritation,† Morbillinum – Catarrh, Coryza, Cough, Fatigue, Chills, Sweats, and Minor Skin Irritation,† Parotidinum – Catarrh, Chills, Sweats and Fatigue,† Rubella Nosode – Headache, Minor Aches of Muscles and Joints, Chills and Sweats,† Salmonella Typhi Nosode – Nausea, Chills and Sweats,† Serum Anguillae – Headache, Fatigue, Support of Kidney, and Minor Joint Aches,† Silicea – Coryza, Cough, Fatigue, Headache, and Minor Joint Aches,† Thuja Occidentalis – Catarrh, Headache, and Minor Joint Aches,† Yersinia Enterocolitica – Chills, Sweats, and Fatigue†
†Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
WARNINGS SECTION
WARNINGS:
If pregnant or breast-feeding, ask a health professional before use.
In case of overdose, get medical help or contact a Poison Control Center right away.
If condition worsens, seek medical attention.
KEEP OUT OF REACH OF CHILDREN
Do not use if tamper evident seal is broken or missing.
Store in a cool place after opening
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
KEEP OUT OF REACH OF CHILDREN:
In case of overdose, get medical help or contact a Poison Control Center right away.
DOSAGE & ADMINISTRATION SECTION
DIRECTIONS:
• Adults: 2 to 3 sprays orally, three times daily. Children under twelve one half adult dosage.
• For highly sensitive individuals: best tolerated administration is through imbrication (RUBBING IN) on skin at pulse points or abdomen.
• Do not take within 15 minutes of consuming food, beverage or brushing teeth.
• Consult a physician for use in children under 12 years of age.
INACTIVE INGREDIENT SECTION
INACTIVE INGREDIENTS:
Alcohol USP 20%, Purified Water USP.
OTC - QUESTIONS SECTION
QUESTIONS:
MANUFACTURED EXCLUSIVELY FOR
NUTRITIONAL SPECIALTIES, INC.
PO BOX 97227
PITTSBURGH, PA 15229
www.phpitd.com
1-800-245-1313
