ticagrelor
These highlights do not include all the information needed to use TICAGRELOR TABLETS safely and effectively. See full prescribing information for TICAGRELOR TABLETS. TICAGRELOR tablets, for oral use Initial U.S. Approval: 2011
7cee944f-e2ca-4fc8-bb97-f46ca28728c5
HUMAN PRESCRIPTION DRUG LABEL
Jul 21, 2020
Amneal Pharmaceuticals NY LLC
DUNS: 123797875
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
ticagrelor
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (12)
Drug Labeling Information
INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
1.1 Acute Coronary Syndrome or a History of Myocardial Infarction
Ticagrelor tablets are indicated to reduce the risk of cardiovascular death, myocardial infarction (MI), and stroke in patients with acute coronary syndrome (ACS) or a history of MI. For at least the first 12 months following ACS, it is superior to clopidogrel.
Ticagrelor tablets also reduce the risk of stent thrombosis in patients who have been stented for treatment of ACS [see Clinical Studies (14.1)].
1.2 Coronary Artery Disease but No Prior Stroke or Myocardial Infarction
Ticagrelor tablets are indicated to reduce the risk of a first MI or stroke in patients with coronary artery disease (CAD) at high risk for such events [see Clinical Studies (14.2)]. While use is not limited to this setting, the efficacy of ticagrelor tablets were established in a population with type 2 diabetes mellitus (T2DM).
Ticagrelor tablets are a P2Y12 platelet inhibitor indicated
- to reduce the risk of cardiovascular (CV) death, myocardial infarction (MI), and stroke in patients with acute coronary syndrome (ACS) or a history of MI. For at least the first 12 months following ACS, it is superior to clopidogrel.
Ticagrelor tablets also reduce the risk of stent thrombosis in patients who have been stented for treatment of ACS. (1.1)
- to reduce the risk of a first MI or stroke in patients with coronary artery disease (CAD) at high risk for such events. While use is not limited to this setting, the efficacy of ticagrelor tablets were established in a population with type 2 diabetes mellitus (T2DM). (1.2)
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
4.1 History of Intracranial Hemorrhage
Ticagrelor tablets are contraindicated in patients with a history of intracranial hemorrhage (ICH) because of a high risk of recurrent ICH in this population [see Clinical Studies (14.1), (14.2)].
4.2 Active Bleeding
Ticagrelor tablets are contraindicated in patients with active pathological bleeding such as peptic ulcer or intracranial hemorrhage [see Warnings and Precautions (5.1) and Adverse Reactions (6.1)].
4.3 Hypersensitivity
Ticagrelor tablets are contraindicated in patients with hypersensitivity (e.g., angioedema) to ticagrelor or any component of the product.
- History of intracranial hemorrhage. (4.1)
- Active pathological bleeding. (4.2)
- Hypersensitivity to ticagrelor or any component of the product. (4.3)
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Strong CYP3A Inhibitors
Strong CYP3A inhibitors substantially increase ticagrelor exposure and so increase the risk of dyspnea, bleeding, and other adverse events. Avoid use of strong inhibitors of CYP3A (e.g., ketoconazole, itraconazole, voriconazole, clarithromycin, nefazodone, ritonavir, saquinavir, nelfinavir, indinavir, atazanavir and telithromycin) [see Clinical Pharmacology (12.3)].
7.2 Strong CYP3A Inducers
Strong CYP3A inducers substantially reduce ticagrelor exposure and so decrease the efficacy of ticagrelor. Avoid use with strong inducers of CYP3A (e.g., rifampin, phenytoin, carbamazepine and phenobarbital) [see Clinical Pharmacology (12.3)].
7.3 Aspirin
Use of ticagrelor with aspirin maintenance doses above 100 mg reduced the effectiveness of ticagrelor [see Warnings and Precautions (5.2) and Clinical Studies (14.1)].
7.4 Opioids
As with other oral P2Y12 inhibitors, co-administration of opioid agonists delay and reduce the absorption of ticagrelor and its active metabolite presumably because of slowed gastric emptying [see Clinical Pharmacology (12.3)]. Consider the use of a parenteral anti-platelet agent in acute coronary syndrome patients requiring co-administration of morphine or other opioid agonists.
7.5 Simvastatin, Lovastatin
Ticagrelor increases serum concentrations of simvastatin and lovastatin because these drugs are metabolized by CYP3A4. Avoid simvastatin and lovastatin doses greater than 40 mg [see Clinical Pharmacology (12.3)].
7.6 Digoxin
Ticagrelor inhibits the P-glycoprotein transporter; monitor digoxin levels with initiation of or change in ticagrelor therapy [see Clinical Pharmacology (12.3)].
- Avoid use with strong CYP3A inhibitors or CYP3A inducers. (7.1, 7.2)
- Opioids: Decreased exposure to ticagrelor. Consider use of parenteral anti-platelet agent. (7.4)
- Patients receiving more than 40 mg per day of simvastatin or lovastatin may be at increased risk of statin-related adverse effects. (7.5)
- Monitor digoxin levels with initiation of or any change in ticagrelor. (7.6)
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Acute Coronary Syndrome or a History of Myocardial Infarction
In the management of ACS, initiate ticagrelor tablets treatment with a 180 mg loading dose. Administer 90 mg twice daily during the first year after an ACS event. After one year, administer 60 mg twice daily.
2.2 Coronary Artery Disease but No Prior Stroke or Myocardial Infarction
Administer 60 mg twice daily. For all patients with ACS [see Dosage and Administration (2.1)].
2.3 Administration
Administer ticagrelor tablets with a daily maintenance dose of aspirin of 75 mg to 100 mg [see Warnings and Precautions (5.2) and Clinical Studies (14)]. A patient who misses a dose of ticagrelor tablets should take one tablet (their next dose) at its scheduled time.
For patients who are unable to swallow tablets whole, ticagrelor tablets can be crushed, mixed with water and drunk. The mixture can also be administered via a nasogastric tube (CH8 or greater) [see Clinical Pharmacology (12.3)].
Do not administer ticagrelor tablets with another oral P2Y12 platelet inhibitor.
-
ACS or History of MI
-
In the management of ACS, initiate treatment with 180 mg oral loading dose. Then administer 90 mg twice daily during the first year. After one year, administer 60 mg twice daily. (2.1)
-
Patients with CAD and No Prior Stroke or MI
-
Administer 60 mg twice daily. (2.2)
Use ticagrelor tablets with a daily maintenance dose of aspirin of 75 mg to 100 mg. (2.3, 5.2)
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Ticagrelor and its major metabolite reversibly interact with the platelet P2Y12 ADP-receptor to prevent signal transduction and platelet activation. Ticagrelor and its active metabolite are approximately equipotent.
12.2 Pharmacodynamics
The inhibition of platelet aggregation (IPA) by ticagrelor and clopidogrel was compared in a 6-week study examining both acute and chronic platelet inhibition effects in response to 20 μM ADP as the platelet aggregation agonist.
The onset of IPA was evaluated on Day 1 of the study following loading doses of 180 mg ticagrelor or 600 mg clopidogrel. As shown in Figure 4, IPA was higher in the ticagrelor group at all time points. The maximum IPA effect of ticagrelor was reached at around 2 hours, and was maintained for at least 8 hours.
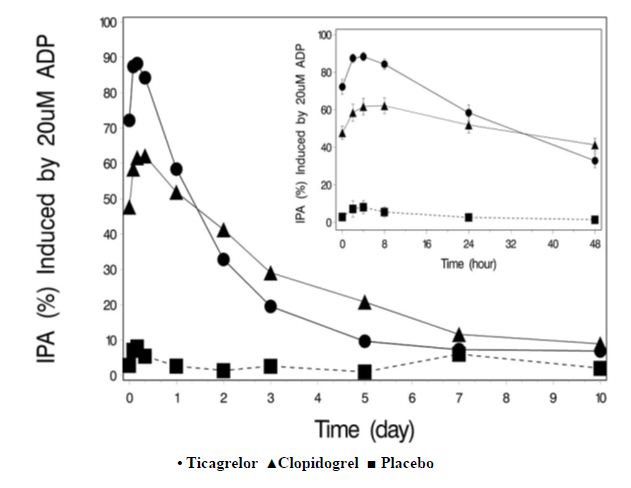
The offset of IPA was examined after 6 weeks on ticagrelor 90 mg twice daily or clopidogrel 75 mg daily, again in response to 20 μM ADP.
As shown in Figure 5, mean maximum IPA following the last dose of ticagrelor was 88% and 62% for clopidogrel. The insert in Figure 5 shows that after 24 hours, IPA in the ticagrelor group (58%) was similar to IPA in clopidogrel group (52%), indicating that patients who miss a dose of ticagrelor would still maintain IPA similar to the trough IPA of patients treated with clopidogrel. After 5 days, IPA in the ticagrelor group was similar to IPA in the placebo group. It is not known how either bleeding risk or thrombotic risk track with IPA, for either ticagrelor or clopidogrel.
Figure 4: Mean inhibition of platelet aggregation (±SE) following single oral doses of placebo, 180 mg ticagrelor or 600 mg clopidogrel
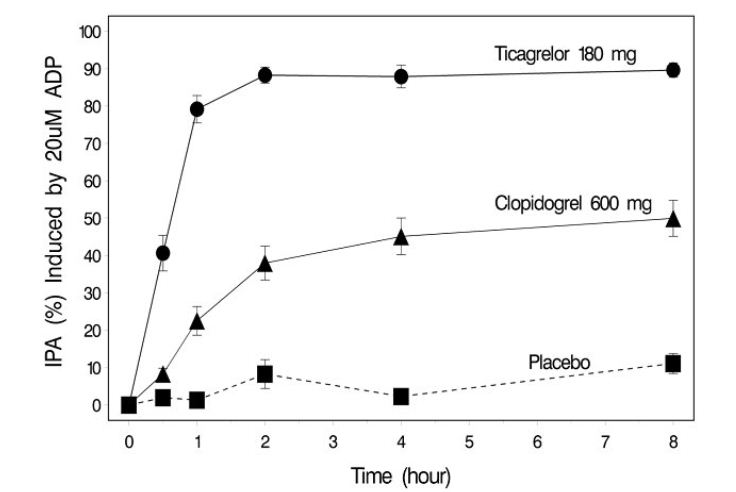
Figure 5: Mean inhibition of platelet aggregation (IPA) following 6 weeks on
placebo, ticagrelor 90 mg twice daily, or clopidogrel 75 mg daily
****
Transitioning from clopidogrel to ticagrelor resulted in an absolute IPA increase of 26.4% and from ticagrelor to clopidogrel resulted in an absolute IPA decrease of 24.5%. Patients can be transitioned from clopidogrel to ticagrelor without interruption of antiplatelet effect [see Dosage and Administration (2)].
12.3 Pharmacokinetics
Ticagrelor demonstrates dose proportional pharmacokinetics, which are similar in patients and healthy volunteers.
Absorption
Ticagrelor tablets can be taken with or without food. Absorption of ticagrelor occurs with a median tmax of 1.5 h (range 1.0 to 4.0). The formation of the major circulating metabolite AR-C124910XX (active) from ticagrelor occurs with a median tmax of 2.5 h (range 1.5 to 5.0).
The mean absolute bioavailability of ticagrelor is about 36% (range 30% to 42%). Ingestion of a high-fat meal had no effect on ticagrelor Cmax, but resulted in a 21% increase in AUC. The Cmax of its major metabolite was decreased by 22% with no change in AUC.
Ticagrelor as crushed tablets mixed in water, given orally or administered through a nasogastric tube into the stomach, is bioequivalent to whole tablets (AUC and Cmax within 80% to 125% for ticagrelor and AR-C124910XX) with a median tmax of 1.0 hour (range 1.0 to 4.0) for ticagrelor and 2.0 hours (range 1.0 to 8.0) for AR-C124910XX.
Distribution
The steady-state volume of distribution of ticagrelor is 88 L. Ticagrelor and the active metabolite are extensively bound to human plasma proteins (> 99%).
Metabolism
CYP3A4 is the major enzyme responsible for ticagrelor metabolism and the formation of its major active metabolite. Ticagrelor and its major active metabolite are weak P-glycoprotein substrates and inhibitors. The systemic exposure to the active metabolite is approximately 30% to 40% of the exposure of ticagrelor.
Excretion
The primary route of ticagrelor elimination is hepatic metabolism. When radiolabeled ticagrelor is administered, the mean recovery of radioactivity is approximately 84% (58% in feces, 26% in urine). Recoveries of ticagrelor and the active metabolite in urine were both less than 1% of the dose. The primary route of elimination for the major metabolite of ticagrelor is most likely to be biliary secretion. The mean t1/2 is approximately 7 hours for ticagrelor and 9 hours for the active metabolite.
Specific Populations
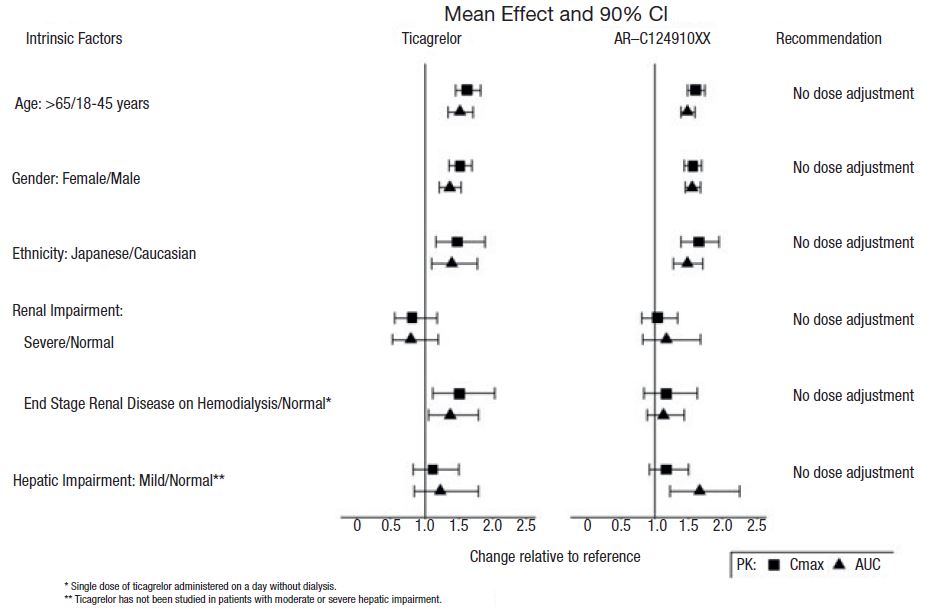
The effects of age, gender, ethnicity, renal impairment and mild hepatic impairment on the pharmacokinetics of ticagrelor are presented in Figure 6. Effects are modest and do not require dose adjustment.
Patients with End-Stage Renal Disease on Hemodialysis
In patients with end stage renal disease on hemodialysis AUC and Cmax of ticagrelor 90 mg administered on a day without dialysis were 38% and 51% higher respectively, compared to subjects with normal renal function. A similar increase in exposure was observed when ticagrelor was administered immediately prior to dialysis showing that ticagrelor is not dialyzable. Exposure of the active metabolite increased to a lesser extent. The IPA effect of ticagrelor was independent of dialysis in patients with end stage renal disease and similar to healthy adults with normal renal function.
Figure 6: Impact of intrinsic factors on the pharmacokinetics of
ticagrelor
****
Effects of Other Drugs on Ticagrelor
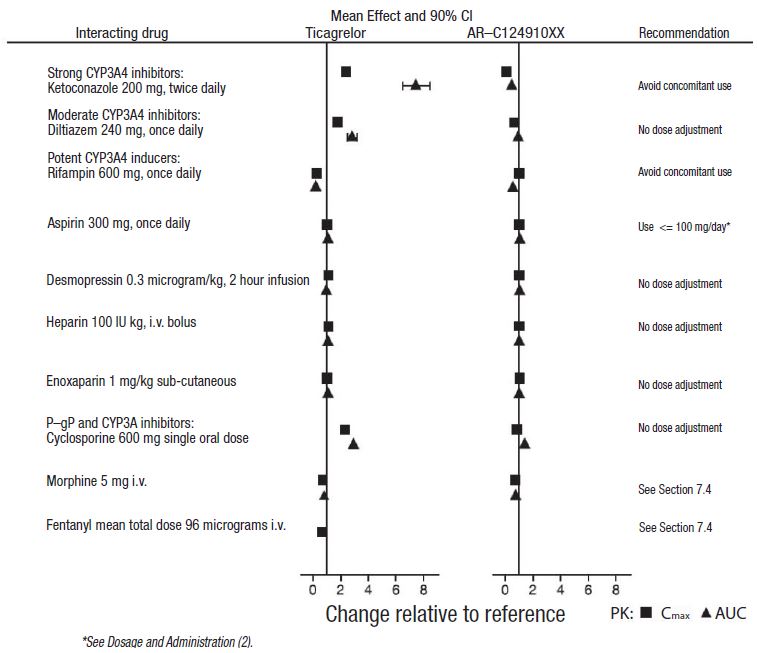
CYP3A4 is the major enzyme responsible for ticagrelor metabolism and the formation of its major active metabolite. The effects of other drugs on the pharmacokinetics of ticagrelor are presented in Figure 7 as change relative to ticagrelor given alone (test/reference). Strong CYP3A inhibitors (e.g., ketoconazole, itraconazole, and clarithromycin) substantially increase ticagrelor exposure. Moderate CYP3A inhibitors have lesser effects (e.g., diltiazem). CYP3A inducers (e.g., rifampin) substantially reduce ticagrelor blood levels. P-gp inhibitors (e.g., cyclosporine) increase ticagrelor exposure.
Co-administration of 5 mg intravenous morphine with 180 mg loading dose of ticagrelor decreased observed mean ticagrelor exposure by up to 25% in healthy adults and up to 36% in ACS patients undergoing PCI. Tmax was delayed by 1 to 2 hours. Exposure of the active metabolite decreased to a similar extent. Morphine co-administration did not delay or decrease platelet inhibition in healthy adults. Mean platelet aggregation was higher up to 3 hours post loading dose in ACS patients co-administered with morphine.
Co-administration of intravenous fentanyl with 180 mg loading dose of ticagrelor in ACS patients undergoing PCI resulted in similar effects on ticagrelor exposure and platelet inhibition.
Figure 7: Effect of co-administered drugs on the pharmacokinetics of ticagrelor
Effects of Ticagrelor on Other Drugs
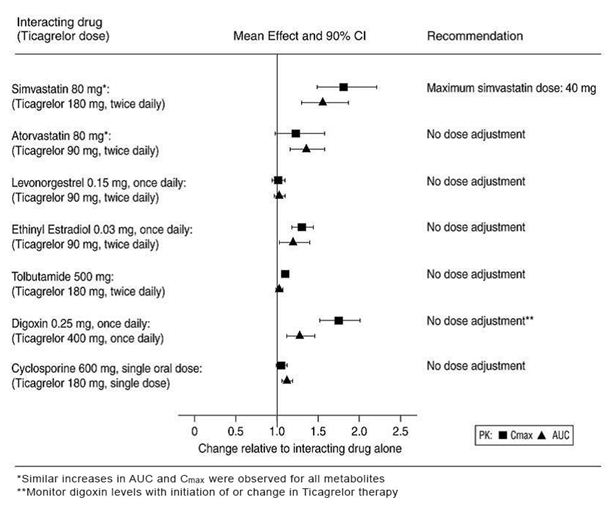
In vitro metabolism studies demonstrate that ticagrelor and its major active metabolite are weak inhibitors of CYP3A4, potential activators of CYP3A5 and inhibitors of the P-gp transporter. Ticagrelor and AR-C124910XX were shown to have no inhibitory effect on human CYP1A2, CYP2C19, and CYP2E1 activity. For specific in vivo effects on the pharmacokinetics of simvastatin, atorvastatin, ethinyl estradiol, levonorgesterol, tolbutamide, digoxin and cyclosporine, see Figure 8.
Figure 8: Impact of ticagrelor on the pharmacokinetics of co-administered drugs
12.5 Pharmacogenetics
In a genetic substudy cohort of PLATO, the rate of thrombotic CV events in the ticagrelor arm did not depend on CYP2C19 loss of function status.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Ticagrelor was not carcinogenic in the mouse at doses up to 250 mg/kg/day or in the male rat at doses up to 120 mg/kg/day (19 and 15 times the MRHD of 90 mg twice daily on the basis of AUC, respectively). Uterine carcinomas, uterine adenocarcinomas and hepatocellular adenomas were seen in female rats at doses of 180 mg/kg/day (29-fold the maximally recommended dose of 90 mg twice daily on the basis of AUC), whereas 60 mg/kg/day (8-fold the MRHD based on AUC) was not carcinogenic in female rats.
Mutagenesis
Ticagrelor did not demonstrate genotoxicity when tested in the Ames bacterial mutagenicity test, mouse lymphoma assay and the rat micronucleus test. The active O-demethylated metabolite did not demonstrate genotoxicity in the Ames assay and mouse lymphoma assay.
Impairment of Fertility
Ticagrelor had no effect on male fertility at doses up to 180 mg/kg/day or on female fertility at doses up to 200 mg/kg/day (> 15-fold the MRHD on the basis of AUC). Doses of ≥ 10 mg/kg/day given to female rats caused an increased incidence of irregular duration estrus cycles (1.5-fold the MRHD based on AUC).
