Ivermectin
These highlights do not include all the information needed to use IVERMECTIN CREAM safely and effectively. See full prescribing information for IVERMECTIN CREAM.IVERMECTIN cream, for topical useInitial U.S. Approval: 1996
1d3e7aea-02a4-46b1-8a14-0324e4777cb4
HUMAN PRESCRIPTION DRUG LABEL
Oct 31, 2022
Padagis Israel Pharmaceuticals Ltd
DUNS: 600093611
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Ivermectin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (17)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
Rx Only
Ivermectin Cream, 1%
For Topical Use Only
Keep Out of Reach of Children
NET WT 45 g
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
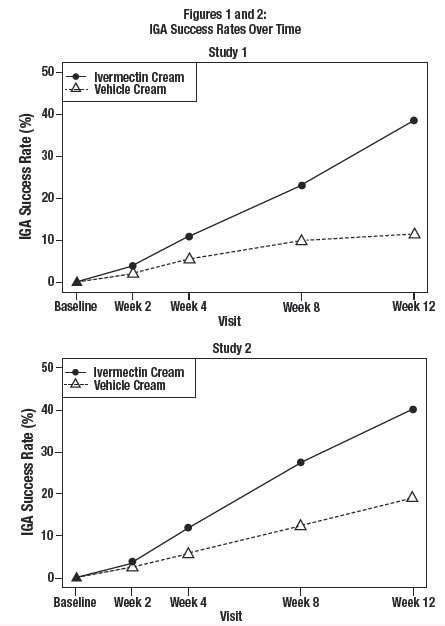
Ivermectin cream applied once daily at bedtime was evaluated in the treatment of inflammatory lesions of rosacea in two randomized, double-blind, vehicle- controlled clinical trials, which were identical in design. The trials were conducted in 1371 subjects aged 18 years and older who were treated once daily for 12 weeks with either ivermectin cream or vehicle cream.
Overall, 96% of subjects were Caucasian and 67% were female. Using the 5-point Investigator Global Assessment (IGA) scale (0=clear, 1=almost clear, 2=mild, 3=moderate, 4=severe), 79% of subjects were scored as moderate (IGA=3) and 21% scored as severe (IGA= 4) at baseline.
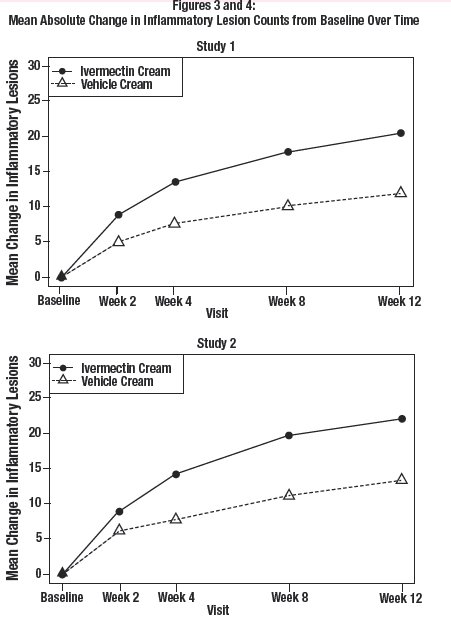
The co-primary efficacy endpoints in both pivotal trials were the success rate based on the IGA outcome (percentage of subjects “clear” and “almost clear”) and absolute change from baseline in inflammatory lesion counts at Week 12. Table 1 presents the co-primary efficacy results at Week 12. Ivermectin cream was more effective than vehicle cream on the co-primary efficacy endpoints starting from 4 weeks of treatment in both studies, see Figures 1 through 4.
|
Table 1: Co-Primary Efficacy Results at Week 12 | ||||
|
Study 1 |
Study 2 | |||
|
Ivermectin Cream (N=451) |
Vehicle Cream (N=232) |
Ivermectin Cream (N=459) |
Vehicle Cream (N=229) | |
|
Investigator Global Assessment: Number (%) of Subjects Clear or Almost Clear |
173 (38.4%) |
27 (11.6%) |
184 (40.1%) |
43 (18.8%) |
|
Inflammatory Lesion Counts: Mean Absolute (%) Change from Baseline |
20.5 (64.9%) |
12.0 (41.6%) |
22.2 (65.7%) |
13.4 (43.4%) |
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Instructions for Use).
Patients using ivermectin cream should receive the following instruction:
Keep out of reach of children.
Manufactured by Padagis®, Yeruham, Israel
www.padagis.com
Rev 03-24
3Y100 RC PH4
SPL UNCLASSIFIED SECTION
Instructions for Use
Ivermectin (eye-ver-MEK-tin) Cream, 1%
**Important:**Ivermectin cream is for use on the skin only (topical use). Do not use ivermectin cream in your mouth, eyes, or vagina. Read and follow the steps below so that you use ivermectin cream correctly.
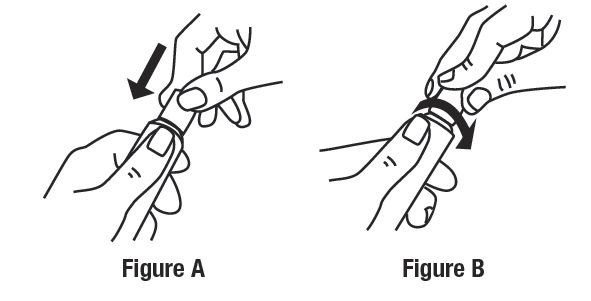
Open the tube of ivermectin cream by gently pressing down on the child resistant cap and twist in the direction of the arrow (counterclockwise) as shown below. See Figures A and B. To avoid spilling, do not squeeze the tube while opening or closing.
To apply ivermectin cream to your face, squeeze a pea-sized amount of ivermectin cream from the tube onto your fingertip. See Figure C.
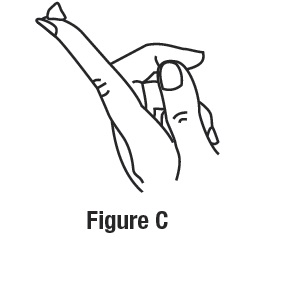
Apply ivermectin cream to the affected areas of your face 1 time a day. Use a pea-sized amount of ivermectin cream for each area of your face (forehead, chin, nose, each cheek) that is affected. Spread the cream smoothly and evenly in a thin layer. Avoid contact with your eyes and lips.
To close ivermectin cream, gently press down on the child resistant cap and twist to the right (clockwise). See Figure D.
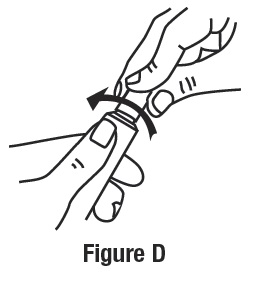
How should I store ivermectin cream?
•
Store ivermectin cream at room temperature between 68°F to 77°F (20°C to 25°C).
Keep ivermectin cream and all medicines out of the reach of children.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by Padagis®
Yeruham, Israel
www.padagis.com
Rev 03-24
3Y100 RC PH4
