Methocarbamol
Methocarbamol Tablets, USP
8cf27774-82ed-4ed1-a55d-41bf476745db
HUMAN PRESCRIPTION DRUG LABEL
Feb 5, 2024
Bryant Ranch Prepack
DUNS: 171714327
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Methocarbamol
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (4)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Methocarbamol Oral Tablet 500 MG #100

INDICATIONS & USAGE SECTION
INDICATIONS AND USAGE
Methocarbamol tablets, USP are indicated as an adjunct to rest, physical therapy, and other measures for the relief of discomfort associated with acute, painful musculoskeletal conditions. The mode of action of methocarbamol has not been clearly identified, but may be related to its sedative properties. Methocarbamol does not directly relax tense skeletal muscles in man.
CONTRAINDICATIONS SECTION
CONTRAINDICATIONS
Methocarbamol tablets, USP are contraindicated in patients hypersensitive to methocarbamol or to any of the tablet components.
ADVERSE REACTIONS SECTION
ADVERSE REACTIONS
Adverse reactions reported coincident with the administration of methocarbamol include:
Body as a whole:
Anaphylactic reaction, angioneurotic edema, fever, headache
Cardiovascular system:
Bradycardia, flushing, hypotension, syncope, thrombophlebitis
Digestive system:
Dyspepsia, jaundice (including cholestatic jaundice), nausea and vomiting
Hemic and lymphatic system:
Leukopenia
Immune system:
Hypersensitivity reactions
Nervous system:
Amnesia, confusion, diplopia, dizziness or lightheadedness, drowsiness, insomnia, mild muscular incoordination, nystagmus, sedation, seizures (including grand mal), vertigo
Skin and special senses:
Blurred vision, conjunctivitis, nasal congestion, metallic taste, pruritus, rash, urticaria
CLINICAL PHARMACOLOGY SECTION
CLINICAL PHARMACOLOGY
The mechanism of action of methocarbamol in humans has not been established, but may be due to general central nervous system (CNS) depression. It has no direct action on the contractile mechanism of striated muscle, the motor end plate or the nerve fiber.
SPL UNCLASSIFIED SECTION
Distributed by:
Solco Healthcare U.S., LLC
Somerset, NJ 08873, USA
Manufactured by:
Prinston Laboratories
3241 Woodpark Blvd, Charlotte, NC 28206
Revised: 06/2022
9040321-05
Rx only
DESCRIPTION SECTION
DESCRIPTION
Methocarbamol tablets, USP, a carbamate derivative of guaifenesin, are a central nervous system (CNS) depressant with sedative and musculoskeletal relaxant properties.
The chemical name of methocarbamol is 3-(2-meth-oxyphenoxy)-1,2-propanediol 1-carbamate and has the empirical formula C11H15NO5. Its molecular weight is 241.24. The structural formula is shown below.
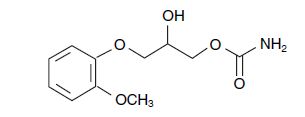
Methocarbamol is a white powder, sparingly soluble in water and chloroform, soluble in alcohol (only with heating) and propylene glycol, and insoluble in benzene and n-hexane.
Methocarbamol tablets, USP are available as 500 mg and 750 mg tablets for oral administration. Methocarbamol tablets, USP 500 mg and 750 mg contain the following inactive ingredients: povidone, sodium starch glycolate and magnesium stearate.
PHARMACOKINETICS SECTION
Pharmacokinetics
In healthy volunteers, the plasma clearance of methocarbamol ranges between 0.20 and 0.80 L/h/kg, the mean plasma elimination half-life ranges between 1 and 2 hours, and the plasma protein binding ranges between 46% and 50%.
Methocarbamol is metabolized via dealkylation and hydroxylation. Conjugation of methocarbamol also is likely. Essentially all methocarbamol metabolites are eliminated in the urine. Small amounts of unchanged methocarbamol also are excreted in the urine.
DOSAGE & ADMINISTRATION SECTION
DOSAGE AND ADMINISTRATION
Methocarbamol tablets, USP, 500 mg – Adults:
Initial dosage: 3 tablets q.i.d.
Maintenance dosage: 2 tablets q.i.d.
Methocarbamol tablets, USP: 750 mg – Adults:
Initial dosage: 2 tablets q.i.d.
Maintenance dosage: 1 tablet q.4h. or 2 tablets t.i.d.
Six grams a day are recommended for the first 48 to 72 hours of treatment. (For severe conditions 8 grams a day may be administered). Thereafter, the dosage can usually be reduced to approximately 4 grams a day.
HOW SUPPLIED SECTION
HOW SUPPLIED
Methocarbamol tablets, USP 500 mg tablets are round standard convex, scored,
white to off-white tablet, debossed S 225 on one side and plain on the reverse
side.
NDC: 72162-1363-1: 100 Tablets in a BOTTLE
NDC: 72162-1363-5: 500 Tablets in a BOTTLE
Store at controlled room temperature, between 20°C and 25°C (68°F and 77°F).
[see USP Controlled Room Temperature].
Dispense in tight container.
Repackaged/Relabeled by:
Bryant Ranch Prepack, Inc.
Burbank, CA 91504
WARNINGS SECTION
WARNINGS
Since methocarbamol may possess a general CNS depressant effect, patients receiving Methocarbamol tablets, USP should be cautioned about combined effects with alcohol and other CNS depressants.
Safe use of Methocarbamol tablets, USP has not been established with regard to possible adverse effects upon fetal development. There have been reports of fetal and congenital abnormalities following in utero exposure to methocarbamol. Therefore, Methocarbamol tablets, USP should not be used in women who are or may become pregnant and particularly during early pregnancy unless in the judgment of the physician the potential benefits outweigh the possible hazards (see**Precautions,**Pregnancy).
Use In Activities Requiring Mental Alertness
Methocarbamol may impair mental and/or physical abilities required for performance of hazardous tasks, such as operating machinery or driving a motor vehicle. Patients should be cautioned about operating machinery, including automobiles, until they are reasonably certain that methocarbamol therapy does not adversely affect their ability to engage in such activities.
PRECAUTIONS SECTION
PRECAUTIONS
Information for patients
Patients should be cautioned that methocarbamol may cause drowsiness or dizziness, which may impair their ability to operate motor vehicles or machinery.
Because methocarbamol may possess a general CNS-depressant effect, patients should be cautioned about combined effects with alcohol and other CNS depressants.
Drug interactions
SeeWarnings andPrecautions for interaction with CNS drugs and alcohol.
Methocarbamol may inhibit the effect of pyridostigmine bromide. Therefore, methocarbamol should be used with caution in patients with myasthenia gravis receiving anticholinesterase agents.
Drug/laboratory test interactions
Methocarbamol may cause a color interference in certain screening tests for 5-hydroxyindoleacetic acid (5-HIAA) using nitrosonaphthol reagent and in screening tests for urinary vanillylmandelic acid (VMA) using the Gitlow method.
Carcinogenesis, mutagenesis, impairment of fertility
Long-term studies to evaluate the carcinogenic potential of methocarbamol have not been performed. No studies have been conducted to assess the effect of methocarbamol on mutagenesis or its potential to impair fertility.
Pregnancy
Teratogenic effects - Pregnancy Category C
Animal reproduction studies have not been conducted with methocarbamol. It is also not known whether methocarbamol can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Methocarbamol tablets, USP should be given to a pregnant woman only if clearly needed.
Safe use of Methocarbamol tablets, USP has not been established with regard to possible adverse effects upon fetal development. There have been reports of fetal and congenital abnormalities following in utero exposure to methocarbamol. Therefore, Methocarbamol tablets, USP should not be used in women who are or may become pregnant and particularly during early pregnancy unless in the judgment of the physician the potential benefits outweigh the possible hazards (seeWarnings).
Nursing mothers
Methocarbamol and/or its metabolites are excreted in the milk of dogs; however, it is not known whether methocarbamol or its metabolites are excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Methocarbamol tablets, USP are administered to a nursing woman.
Pediatric use
Safety and effectiveness of Methocarbamol tablets, USP in pediatric patients below the age of 16 have not been established.
OVERDOSAGE SECTION
OVERDOSAGE
Limited information is available on the acute toxicity of methocarbamol. Overdose of methocarbamol is frequently in conjunction with alcohol or other CNS depressants and includes the following symptoms: nausea, drowsiness, blurred vision, hypotension, seizures, and coma.
In post-marketing experience, deaths have been reported with an overdose of methocarbamol alone or in the presence of other CNS depressants, alcohol or psychotropic drugs.
Treatment
Management of overdose includes symptomatic and supportive treatment. Supportive measures include maintenance of an adequate airway, monitoring urinary output and vital signs, and administration of intravenous fluids if necessary. The usefulness of hemodialysis in managing overdose is unknown.
