Oxybutynin Chloride
Oxybutynin Chloride Extended-Release Tablets USP Rx Only These highlights do not include all the information needed to use Oxybutynin chloride extended-release tablets safely and effectively. See full prescribing information for Oxybutynin chloride extended-release tablets. Oxybutynin chloride extended-release tablets for oral use Initial U.S. Approval: 1975
02ff3be7-fc5c-4b91-8c67-ecdf6e19c42a
HUMAN PRESCRIPTION DRUG LABEL
May 31, 2023
Aphena Pharma Solutions - Tennessee, LLC
DUNS: 128385585
Products 3
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Oxybutynin Chloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (15)
Oxybutynin Chloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (15)
Oxybutynin Chloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (15)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 15 mg
NDC 43353-322 - Oxybutynin Chloride ER 15 mg - Rx Only

WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Angioedema
Angioedema of the face, lips, tongue and/or larynx has been reported with oxybutynin. In some cases, angioedema occurred after the first dose. Angioedema associated with upper airway swelling may be life-threatening. If involvement of the tongue, hypopharynx, or larynx occurs, oxybutynin should be promptly discontinued and appropriate therapy and/or measures necessary to ensure a patent airway should be promptly provided.
5.2 Central Nervous System Effects
Oxybutynin is associated with anticholinergic central nervous system (CNS) effects [see Adverse Reactions (6)]. A variety of CNS anticholinergic effects have been reported, including hallucinations, agitation, confusion and somnolence. Patients should be monitored for signs of anticholinergic CNS effects, particularly in the first few months after beginning treatment or increasing the dose. Advise patients not to drive or operate heavy machinery until they know how Oxybutynin chloride extended-release tablets affect them. If a patient experiences anticholinergic CNS effects, dose reduction or drug discontinuation should be considered.
Oxybutynin chloride extended-release tablets should be used with caution in patients with preexisting dementia treated with cholinesterase inhibitors due to the risk of aggravation of symptoms.
Oxybutynin chloride extended-release tablets should be used with caution in patients with Parkinson's disease due to the risk of aggravation of symptoms.
5.3 Worsening of Symptoms of Myasthenia Gravis
Oxybutynin chloride extended-release tablets should be used with caution in patients with myasthenia gravis due to the risk of symptom aggravation.
5.4 Worsening of Symptoms of Decreased Gastrointestinal Motility in
Patients with Autonomic Neuropathy
Oxybutynin chloride extended-release tablets should be used with caution in patients with autonomic neuropathy due to the risk of aggravation of symptoms of decreased gastrointestinal motility.
5.5 Urinary Retention
Oxybutynin chloride extended-release tablets should be administered with caution to patients with clinically significant bladder outflow obstruction because of the risk of urinary retention [see Contraindications (4)].
5.6 Gastrointestinal Adverse Reactions
Oxybutynin chloride extended-release tablets should be administered with caution to patients with gastrointestinal obstructive disorders because of the risk of gastric retention [see Contraindications (4)].
Oxybutynin chloride extended-release tablets, like other anticholinergic drugs, may decrease gastrointestinal motility and should be used with caution in patients with conditions such as ulcerative colitis and intestinal atony.
Oxybutynin chloride extended-release tablets should be used with caution in patients who have gastroesophageal reflux and/or who are concurrently taking drugs (such as bisphosphonates) that can cause or exacerbate esophagitis.
As with any other nondeformable material, caution should be used when administering Oxybutynin chloride extended-release tablets to patients with preexisting severe gastrointestinal narrowing (pathologic or iatrogenic). There have been rare reports of obstructive symptoms in patients with known strictures in association with the ingestion of other drugs in nondeformable controlled-release formulations.
- Angioedema: Angioedema has been reported with oxybutynin. If symptoms of angioedema occur, discontinue Oxybutynin chloride extended-release tablets immediately and initiate appropriate therapy. ( 5.1)
- Central Nervous System (CNS) effects: CNS effects have been reported with oxybutynin. If patient experiences anticholinergic CNS effects, consider dose adjustment or discontinuation of Oxybutynin chloride extended-release tablets. ( 5.2)
- Use with caution due to aggravation of symptoms:
- Pre-existing dementia in patients treated with cholinesterase inhibitors ( 5.2),
- Parkinson's disease ( 5.2),
- Myasthenia gravis ( 5.3), and
- Decreased gastrointestinal motility in patients with autonomic neuropathy. ( 5.4)
- Urinary Retention: Use with caution in patients with clinically significant bladder outflow obstruction because of the risk of urinary retention ( 5.5)
- Gastrointestinal Adverse Reactions: Use with caution in patients with gastrointestinal obstructive disorders or decreased intestinal motility due to risk of gastric retention. Use with caution in patients with gastroesophageal reflux or in patients concurrently taking drugs that can exacerbate esophagitis. ( 5.6)
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
Oxybutynin chloride extended-release tablets must be swallowed whole with the aid of liquids, and must not be chewed, divided, or crushed.
Oxybutynin chloride extended-release tablets may be administered with or without food.
2.1 Adults
The recommended starting dose of Oxybutynin chloride extended-release tablets is 5 or 10 mg once daily at approximately the same time each day. Dosage may be adjusted in 5-mg increments to achieve a balance of efficacy and tolerability (up to a maximum of 30 mg/day). In general, dosage adjustment may proceed at approximately weekly intervals.
2.2 Pediatric Patients Aged 6 Years of Age and Older
The recommended starting dose of Oxybutynin chloride extended-release tablets is 5 mg once daily at approximately the same time each day. Dosage may be adjusted in 5-mg increments to achieve a balance of efficacy and tolerability (up to a maximum of 20 mg/day).
Oxybutynin chloride extended-release tablets must be swallowed whole with the aid of liquids, and must not be chewed, divided, or crushed. Oxybutynin chloride extended-release tablets may be administered with or without food. (2)
***Adults:**Start with 5 mg or 10 mg, once daily at approximately the same time every day. Dose should not exceed 30 mg per day. ( 2.1) ***Pediatric patients (6 years of age or older):**Start with 5 mg, once daily at approximately the same time every day. Dose should not exceed 20 mg per day. ( 2.2)
DESCRIPTION SECTION
11 DESCRIPTION
Oxybutynin chloride extended-release tablets are an antispasmodic, muscarinic antagonist. Each Oxybutynin chloride extended-release tablets contains 5 mg, 10 mg, or 15 mg of oxybutynin chloride USP, formulated as a once-a-day controlled-release tablet for oral administration. Oxybutynin chloride is administered as a racemate of R- and S-enantiomers.
Chemically, oxybutynin chloride is d,l (racemic) 4-diethylamino-2-butynyl phenylcyclohexylglycolate hydrochloride. The empirical formula of oxybutynin chloride is C 22H 31NO 3•HCl.
Its structural formula is:
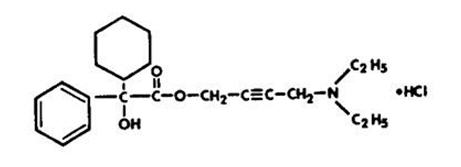
Oxybutynin chloride is a white crystalline solid with a molecular weight of 393.9. It is readily soluble in water and acids, but relatively insoluble in alkalis.
Oxybutynin chloride extended-release tablets also contain the following inert ingredients: lactose, mannitol, dextrose, tartaric acid, colloidal silicon dioxide, magnesium stearate, cellulose acetate, polyethylene glycol, titanium dioxide, triacetin, black iron oxide, propylene glycol, hypromellose.
System Components and Performance
Oxybutynin chloride extended-release tablets uses osmotic pressure to deliver oxybutynin chloride at a controlled rate over approximately 24 hours. The system, which resembles a conventional tablet in appearance, comprises an osmotically active core surrounded by a semipermeable membrane. The unitary tablet core is composed of the drug and excipients (including the osmotically active components). There is a precision-laser drilled orifice in the semipermeable membrane on the side of the tablet. In an aqueous environment, such as the gastrointestinal tract, water permeates through the membrane into the tablet core, causing the drug to go into suspension and the osmotic components to expand. This expansion pushes the drug out through the orifice. The semipermeable membrane controls the rate at which water permeates into the tablet core, which in turn controls the rate of drug delivery. The controlled rate of drug delivery into the gastrointestinal lumen is thus independent of pH or gastrointestinal motility. The function of Oxybutynin chloride extended- release tablets depends on the existence of an osmotic gradient between the contents of the core and the fluid in the gastrointestinal tract. Since the osmotic gradient remains constant, drug delivery remains essentially constant. The biologically inert components of the tablet remain intact during gastrointestinal transit and are eliminated in the feces as an insoluble shell.
USP Drug Release Test 3.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
Oxybutynin chloride extended-release tablets were evaluated for the treatment of patients with overactive bladder with symptoms of urge urinary incontinence, urgency, and frequency in three controlled efficacy studies. The majority of patients were Caucasian (89.0%) and female (91.9%) with a mean age of 59 years (range, 18 to 98 years). Entry criteria required that patients have urge or mixed incontinence (with a predominance of urge) as evidenced by ≥ 6 urge incontinence episodes per week and ≥ 10 micturitions per day. Study 1 was a fixed-dose escalation design, whereas the other two studies used a dose- adjustment design in which each patient's final dose was adjusted to a balance between improvement of incontinence symptoms and tolerability of side effects. All three studies included patients known to be responsive to oxybutynin or other anticholinergic medications, and these patients were maintained on a final dose for up to 2 weeks.
The efficacy results for the three controlled trials are presented in the following tables and figures.
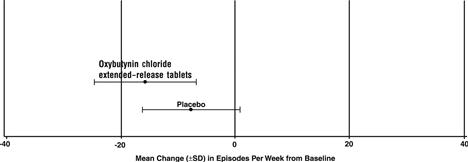
Number of Urge Urinary Incontinence Episodes Per Week|
Study 1 |
n |
Oxybutynin chloride extended-release tablets |
n |
Placebo |
|---|---|---|---|---|
| ||||
|
Mean Baseline |
34 |
15.9 |
16 |
20.9 |
|
Mean (SD) Change from Baseline * |
34 |
-15.8 (8.9) |
16 |
-7.6 (8.6) |
|
95% Confidence Interval for Difference |
(-13.6, -2.8) † | |||
|
(Oxybutynin chloride extended-release tablets- Placebo) |
|
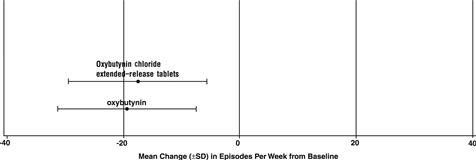
Study 2 |
n |
Oxybutynin chloride extended-release tablets |
n |
oxybutynin |
|---|---|---|---|---|
| ||||
|
Mean Baseline |
53 |
27.6 |
52 |
23.0 |
|
Mean (SD) Change from Baseline * |
53 |
-17.6 (11.9) |
52 |
-19.4 (11.9) |
|
95% Confidence Interval for Difference |
(-2.8, 6.5) | |||
|
(Oxybutynin chloride extended-release tablets- oxybutynin) |
|
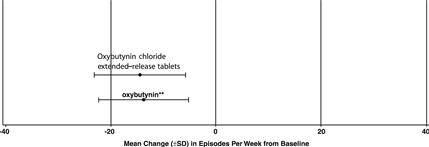
Study 3 |
n |
Oxybutynin chloride extended-release tablets |
n |
oxybutynin |
|---|---|---|---|---|
| ||||
|
Mean Baseline |
111 |
18.9 |
115 |
19.5 |
|
Mean (SD) Change from Baseline * |
111 |
-14.5 (8.7) |
115 |
-13.8 (8.6) |
|
95% Confidence Interval for Difference |
(-3.0, 1.6) † | |||
|
(Oxybutynin chloride extended-release tablets- oxybutynin) |
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Oxybutynin chloride extended-release tablets 5 mg are round, biconvex, white coated tablets imprinted in black ink with "270" on one side and "KU" on the other side.
They are supplied as follows:
Bottles of 100 Tablets NDC 42291-633-01
Bottles of 500 Tablets NDC 42291-633-50
Oxybutynin chloride extended-release tablets 10 mg are round, biconvex, white coated tablets imprinted in black ink with "271" on one side and "KU" on the other side.
They are supplied as follows:
Bottles of 100 Tablets NDC 42291-634-01
Bottles of 500 Tablets NDC 42291-634-50
Oxybutynin chloride extended-release tablets 15 mg are round, biconvex, white coated tablets imprinted in black ink with "272" on one side and "KU" on the other side.
They are supplied as follows:
Bottles of 100 Tablets NDC 42291-635-01
16.1 Storage
Store at 25°C (77°F); excursions permitted to 15–30°C (59–86°F) [see USP Controlled Room Temperature]. Protect from moisture and humidity.
SPL UNCLASSIFIED SECTION
Repackaging Information
Please reference theHow Supplied section listed above for a description of individual tablets. This drug product has been received by Aphena Pharma - TN in a manufacturer or distributor packaged configuration and repackaged in full compliance with all applicable cGMP regulations. The package configurations available from Aphena are listed below:
|
Count |
5 mg |
10 mg |
15 mg |
|
30 |
43353-282-30 |
43353-284-30 |
|
|
60 |
43353-282-53 |
|
|
|
90 |
43353-282-60 |
43353-284-60 |
|
|
180 |
43353-282-80 |
|
|
|
6000 |
|
|
43353-322-16 |
Store between 20°-25°C (68°-77°F). See USP Controlled Room Temperature. Dispense in a tight light-resistant container as defined by USP. Keep this and all drugs out of the reach of children.
Repackaged by:

Cookeville, TN 38506
20230531JK
