Synarel
Synarel (nafarelin acetate) nasal solution
d0aa57cb-d2f4-46d7-af43-7c8b06aa81a6
HUMAN PRESCRIPTION DRUG LABEL
Feb 22, 2023
Pfizer Laboratories Div Pfizer Inc
DUNS: 134489525
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
nafarelin acetate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Drug Labeling Information
WARNINGS SECTION
WARNINGS
Safe use of nafarelin acetate in pregnancy has not been established clinically. Before starting treatment with SYNAREL, pregnancy must be excluded.
When used regularly at the recommended dose, SYNAREL usually inhibits ovulation and stops menstruation. Contraception is not insured, however, by taking SYNAREL, particularly if patients miss successive doses. Therefore, patients should use nonhormonal methods of contraception. Patients should be advised to see their physician if they believe they may be pregnant. If a patient becomes pregnant during treatment, the drug must be discontinued and the patient must be apprised of the potential risk to the fetus.
Clinical Depression
Depression may occur or worsen during treatment with GnRH agonists including SYNAREL 2 mg/mL. Carefully observe women for depression, especially those with a history of depression and consider whether the risks of continuing SYNAREL 2 mg/mL outweigh the benefits. Women with new or worsening depression should be referred to a mental health professional, as appropriate [see Precautions].
HOW SUPPLIED SECTION
HOW SUPPLIED
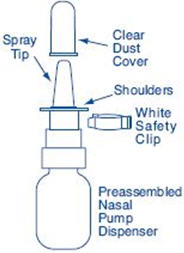
Each 0.5 ounce bottle (NDC 0025-0166-08) contains 8 mL SYNAREL (nafarelin acetate) Nasal Solution 2 mg/mL (as nafarelin base), and is supplied with a metered spray pump that delivers 200 µg of nafarelin per spray. A dust cover and a leaflet of patient instructions are also included.
Store upright at 25°C (77°F); excursions permitted to 15–30°C (59–86°F) [see USP Controlled Room Temperature]. Protect from light.
SPL PATIENT PACKAGE INSERT SECTION
Instructions for Use
SYNAREL(sin-na-rell)
(nafarelin acetate)
nasal solution
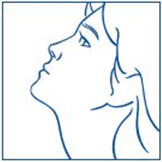
For use in the nose only.
|
Figure A |
|
|
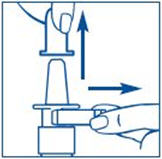
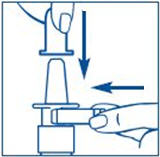
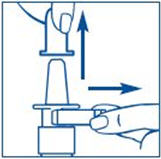
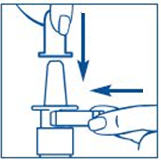
Before you use SYNAREL nasal spray for the first time, you will need to prime it. This will make sure that you get the right dose of medicine each time you use it. Priming only needs to be done 1 time, when you start using a new bottle of SYNAREL.
To Prime the Pump:
|
Figure B |
|
|
Figure C |
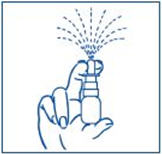
It is normal to see some larger droplets of liquid within the fine mist. However, if SYNAREL comes out of the pump as a thin stream of liquid instead of a fine mist, SYNAREL may not work as well, and you should talk to your pharmacist. |
|
Figure D |
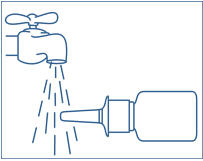
• Rinse the "spray tip" withwarm water while wiping the tip with your finger or soft cloth for 15 seconds. • • • • |
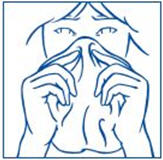
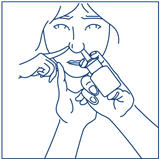
How to use the SYNAREL Nasal Spray for the treatment of Central Precocious Puberty
|
Figure F |
|
|
Figure G |
• • • • • |
|
Figure H |
|
|
Figure I |
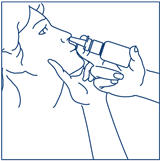
Put pressureevenly to the "shoulders" and push downquickly and firmly. Pump the sprayer 1 time, at the same time as the child sniffs in gently. Wait about 30 seconds and put one more spray in the same nostril. Repeat this process in the other nostril, for a total of four sprays. If the sprayer fails to deliver the dose, clean the spray tip (See Step 5Clean the Spray Tip each time before and after using SYNAREL). |
|
Figure J |
|
|
Figure K |
|
It is important that you clean the spray tip before and after every use. Not doing this may result in a clogged tip that may cause you to get the wrong dose of medicine.
How should I store SYNAREL?
•
Store SYNAREL at room temperature between 59°F to 86°F (15°C to 30°C).
•
Store the SYNAREL bottle upright.
•
Keep SYNAREL out of the light.
Keep SYNAREL and all medicines out of the reach of children.
For more information call 1-800-438-1985.
This Medication Guide and Instructions for Use have been approved by the U.S. Food and Drug Administration.
Manufactured for: Pfizer Inc., 235 East 42nd Street, New York, NY, 10017

LAB-1049-3.0
Revised: April 2022