Rexall
5820346 DG Max Str OP Relief Liq
8f6e2780-cfb7-4bef-9b7d-13cb80d8923e
HUMAN OTC DRUG LABEL
Aug 12, 2025
Rexall
DUNS: 068331990
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Benzocaine
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
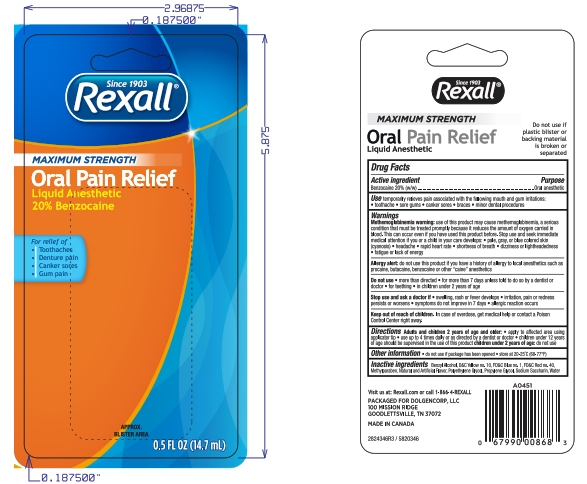
INDICATIONS & USAGE SECTION
Directions
Adults and children 2 years of age and older:
- apply to affect4ed area using applicator tip
- use up to 4 times daily or as directed by a dentist or doctor.
- Children under 12 years of age should be supervised in the use of this product.
Children under 2 years of age: ask a dentist or doctor.
OTC - ACTIVE INGREDIENT SECTION
Active ingredient
Benzocaine 20.0% ..................................Purpose: Oral pain reliever
OTC - PURPOSE SECTION
Uses
Temporarily relieves pain associated with the following mouth irritations toothache sore gums canker sores braces minor dental procedures
WARNINGS SECTION
Allergy alert: do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or other “caine” anesthetics
When using this product
- avoid contact with eyes
- do not exceed recommended dosage
- Do not use more than directed for more than 7 days unless directed by a dentist or doctor.
Stop use and ask a doctor if sore mouth symptoms do not improve in 7 days, swelling, rash or fever develops, irritation, pain or redness persists or worsens swelling rash or fever developes
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
OTHER SAFETY INFORMATION
Other Information
Do not use if package has been opened
Store at 20-25°C (68-77°F)
INACTIVE INGREDIENT SECTION
Inactive ingredients
Benzyl Alcohol, D&C Yellow 10, FD&C Blue 1, FD&C Red 40, FD&C Yellow 5, Methylparaben, Flavor, Polyethylene Glycol, Propylene Glycol, Sodium Saccharin, Water
DOSAGE & ADMINISTRATION SECTION
Adults and children 2 years of age and older:
apply to affect4ed area using applicator tip
use up to 4 times daily or as directed by a dentist or doctor.
Children under 12 years of age should be supervised in the use of this
product.
Children under 2 years of age: ask a dentist or doctor.
