Thymoglobulin
These highlights do not include all the information needed to use THYMOGLOBULIN safely and effectively. See full prescribing information for THYMOGLOBULIN. THYMOGLOBULIN (anti-thymocyte globulin [rabbit]) for injection, for intravenous use Initial U.S. Approval: 1998
bbd8ab99-552e-4b81-aca4-6b0c7af8b9ae
HUMAN PRESCRIPTION DRUG LABEL
Mar 31, 2023
Genzyme Corporation
DUNS: 025322157
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Anti-thymocyte Globulin (Rabbit)
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (4)
Drug Labeling Information
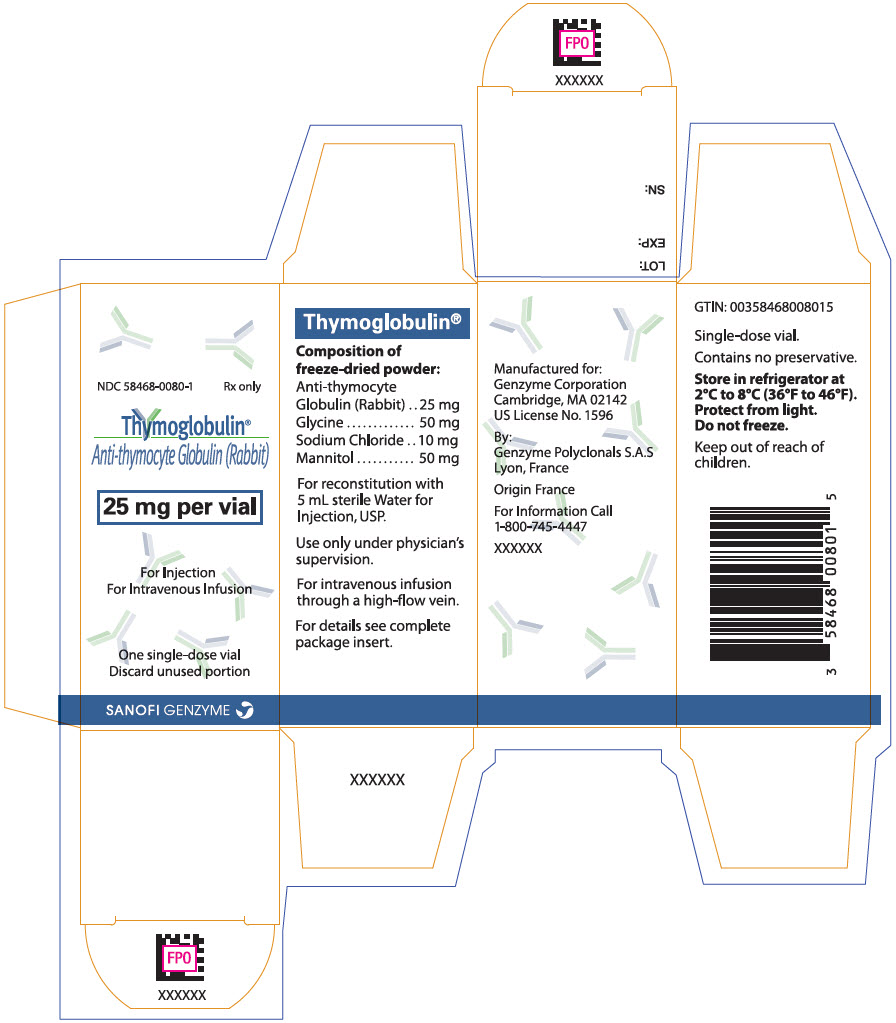
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 25 mg Vial Carton
NDC 58468-0080-1
Rx only
Thymoglobulin®
Anti-thymocyte Globulin (Rabbit)
25 mg per vial
For Injection
For Intravenous Infusion
One single-dose vial
Discard unused portion
SANOFI GENZYME
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
THYMOGLOBULIN is contraindicated in patients with history of allergy or anaphylactic reaction to rabbit proteins or to any product excipients, or who have active acute or chronic infections that contraindicate any additional immunosuppression [see Warnings and Precautions (5.2, 5.5) and Adverse Reactions (6.2)].
Allergy or anaphylactic reaction to rabbit proteins or to any product excipients, or active acute or chronic infections which contraindicate any additional immunosuppression (4)
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Management of Immunosuppression
To prevent over-immunosuppression, physicians may wish to decrease the dose of the maintenance immunosuppression regimen during the period of THYMOGLOBULIN use.
Dosing for THYMOGLOBULIN is different from dosing for other anti-thymocyte globulin (ATG) products, because protein composition and concentrations vary depending on the source of ATG. The prescribing physician must ensure that the dose prescribed is appropriate for the ATG product being administered.
5.2 Immune-Mediated Reactions
Serious immune-mediated reactions, including anaphylaxis or severe cytokine release syndrome (CRS), have been reported with the use of THYMOGLOBULIN [see Warnings and Precautions (5.3)].
Fatal anaphylaxis has been reported. If an anaphylactic reaction occurs, terminate the infusion immediately. Provide emergency treatment, such as 0.3 mL to 0.5 mL aqueous epinephrine (1:1000 dilution) subcutaneously and other resuscitative measures including oxygen, intravenous fluids, antihistamines, corticosteroids, pressor amines, and airway management, as clinically indicated.
5.3 Infusion-Associated Reactions
Cases consistent with cytokine release syndrome (CRS) have been reported with rapid infusion rates. CRS is attributed to the release of cytokines by activated monocytes and lymphocytes. Severe acute CRS can cause serious cardiorespiratory events and/or death [see Adverse Reactions (6.2)]. Close compliance with the recommended dosage and infusion time may reduce the incidence and severity of infusion-associated reactions (IARs). Slowing the infusion rate may minimize many of these IARs.
Reactions at the infusion site may include pain, swelling, and redness of the skin.
5.4 Hematologic Effects
Low counts of platelets and white blood cells (including low counts of lymphocytes and neutrophils) have been identified and are reversible following dose adjustments. Total white blood cell and platelet counts should be monitored [see Dosage and Administration (2.3)].
5.5 Infection
THYMOGLOBULIN is routinely used in combination with other immunosuppressive agents. Infections (bacterial, fungal, viral and protozoal), reactivation of infection (particularly cytomegalovirus [CMV]) and sepsis have been reported after THYMOGLOBULIN administration in combination with multiple immunosuppressive agents. These infections can be fatal.
Monitor patients carefully and administer appropriate anti-infective treatment when indicated [see Dosage and Administration (2.4)].
5.6 Malignancy
Use of immunosuppressive agents, including THYMOGLOBULIN, may increase the incidence of malignancies, including lymphoma or lymphoproliferative disorders. These events have been associated with fatal outcome [see Adverse Reactions (6.2)].
5.7 Immunizations
The safety of immunization with attenuated live vaccines following THYMOGLOBULIN therapy has not been studied; therefore, immunization with attenuated live vaccines is not recommended for patients who have recently received THYMOGLOBULIN.
5.8 Laboratory Tests
THYMOGLOBULIN may interfere with rabbit antibody–based immunoassays and with cross-match or panel-reactive antibody cytotoxicity assays. THYMOGLOBULIN has not been shown to interfere with any routine clinical laboratory tests that do not use immunoglobulins.
- THYMOGLOBULIN should only be used by physicians experienced in immunosuppressant therapy in transplantation. (5.1)
- Immune-mediated reactions: THYMOGLOBULIN infusion could result in an anaphylactic reaction. (5.2)
- Infusion-associated reactions: Close compliance with the recommended infusion time may reduce the incidence and severity of infusion-associated reactions. (5.3)
- Hematologic effects: low counts of platelets and white blood cells have been identified and are reversible following dose adjustments. Monitor total white blood cell and platelet counts. (5.4)
- Infection: Infections and reactivation of infections have been reported. Monitor patients and administer anti-infective prophylaxis. (5.5)
- Malignancy: Incidence of malignancies may increase. (5.6)
- Immunization with attenuated live vaccines is not recommended for patients who have recently received THYMOGLOBULIN. (5.7)
- THYMOGLOBULIN may interfere with rabbit antibody–based immunoassays and with cross-match or panel-reactive antibody cytotoxicity assays. (5.8)
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
THYMOGLOBULIN is supplied as a single-dose clear glass 10 mL vial containing 25 mg of lyophilized (solid) THYMOGLOBULIN. Each carton contains one THYMOGLOBULIN vial (NDC 58468-0080-1).
16.2 Storage and Handling
- Store in refrigerator at 2°C to 8°C (36°F to 46°F).
- Protect from light.
- Do not freeze.
- Do not use after the expiration date indicated on the label.
- Reconstituted THYMOGLOBULIN is physically and chemically stable for up to 24 hours at room temperature; however, room temperature storage is not recommended. As THYMOGLOBULIN contains no preservatives, reconstituted product should be used immediately.
- Infusion solutions of THYMOGLOBULIN must be used immediately.
- Any unused drug remaining after infusion must be discarded.
