HYDROXYCHLOROQUINE SULFATE
These highlights do not include all the information needed to use HYDROXYCHLOROQUINE SULFATE TABLETS safely and effectively. See full prescribing information for HYDROXYCHLOROQUINE SULFATE TABLETS. HYDROXYCHLOROQUINE SULFATE tablets, for oral use Initial U.S. Approval: 1955
452092b4-7a8f-4d19-8113-f1c2a948d3d8
HUMAN PRESCRIPTION DRUG LABEL
Aug 18, 2023
Dr. Reddy's Laboratories Inc.
DUNS: 802315887
Products 4
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
HYDROXYCHLOROQUINE SULFATE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
HYDROXYCHLOROQUINE SULFATE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
HYDROXYCHLOROQUINE SULFATE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
HYDROXYCHLOROQUINE SULFATE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
Drug Labeling Information
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Drugs Prolonging QT Interval and Other Arrhythmogenic Drugs
Hydroxychloroquine sulfate tablets prolongs the QT interval. There may be an increased risk of inducing ventricular arrhythmias if hydroxychloroquine sulfate tablets is used concomitantly with other arrhythmogenic drugs. Therefore, hydroxychloroquine sulfate tablets are not recommended in patients taking other drugs that have the potential to prolong the QT interval or are arrhythmogenic [see Warnings and Precautions (5.1].
7.2 Insulin or Other Antidiabetic Drugs
Hydroxychloroquine sulfate tablets may enhance the effects of insulin and antidiabetic drugs, and consequently increase the hypoglycemic risk. Therefore, a decrease in dosage of insulin and other antidiabetic drugs may be necessary [see Warnings and Precautions (5.10)].
7.3 Drugs that Lower the Seizure Threshold
Hydroxychloroquine sulfate tablets can lower the seizure threshold. Co- administration of hydroxychloroquine sulfate tablets with other antimalarials known to lower the seizure threshold (e.g., mefloquine) may increase the risk of seizures.
7.4 Antiepileptics
The activity of antiepileptic drugs might be impaired if co-administered with hydroxychloroquine sulfate tablets.
7.5 Methotrexate
Concomitant use of hydroxychloroquine sulfate tablets and methotrexate may increase the incidence of adverse reactions.
7.6 Cyclosporine
An increased plasma cyclosporin level was reported when cyclosporin and hydroxychloroquine sulfate tablets were co-administered. Monitor serum cyclosporine levels closely in patients receiving combined therapy.
7.7 Digoxin
Concomitant hydroxychloroquine sulfate tablets and digoxin therapy may result in increased serum digoxin levels. Monitor serum digoxin levels closely in patients receiving combined therapy.
7.8 Cimetidine
Concomitant use of cimetidine resulted in a 2-fold increase of exposure of chloroquine, which is structurally related to hydroxychloroquine. Interaction of cimetidine with hydroxychloroquine cannot be ruled out. Avoid concomitant use of cimetidine.
7.9 Rifampicin
Lack of efficacy of hydroxychloroquine was reported when rifampicin was concomitantly administered. Avoid concomitant use of rifampicin.
7.10 Praziquantel
Chloroquine has been reported to reduce the bioavailability of praziquantel. Interaction of praziquantel with hydroxychloroquine cannot be ruled out.
7.11 Antacids and kaolin
Antacids and kaolin can reduce absorption of chloroquine; an interval of at least 4 hours between intake of these agents and chloroquine should be observed. Interaction of antacids and kaolin with hydroxychloroquine cannot be ruled out.
7.12 Ampicillin
In a study of healthy volunteers, chloroquine significantly reduced the bioavailability of ampicillin. Interaction of ampicillin with hydroxychloroquine cannot be ruled out.
- Drugs Prolonging QT Interval and Other Arrhythmogenic Drugs. (7.1)
- See FPI for more important drug interactions. (7)
DESCRIPTION SECTION
11 DESCRIPTION
Hydroxychloroquine sulfate is a white or practically white, crystalline powder, freely soluble in water; practically insoluble in alcohol, chloroform, and in ether. The chemical name for hydroxychloroquine sulfate is 2-[[4-[(7-Chloro-4-quinolyl)amino]pentyl]ethylamino] ethanol sulfate (1:1). Its structural formula is
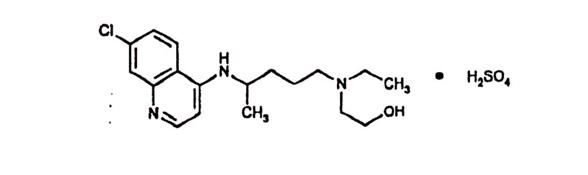
The molecular weight of hydroxychloroquine sulfate is 433.95, and molecular formula is C18H26CIN3O•H2SO4.
Hydroxychloroquine Sulfate Tablets, USP contains 100 mg hydroxychloroquine sulfate, equivalent to 77.5 mg base, and are for oral administration.
Inactive Ingredients: corn starch, crospovidone, hydroxypropyl methylcellulose, lactose monohydrate, magnesium stearate, polyethylene glycol, polyvinyl alcohol, talc and titanium dioxide.
Hydroxychloroquine Sulfate Tablets, USP contain 200 mg hydroxychloroquine sulfate, equivalent to 155 mg base, and are for oral administration.
Inactive Ingredients: corn starch, crospovidone, hydroxypropyl methylcellulose, lactose monohydrate, magnesium stearate, polyethylene glycol, polyvinyl alcohol, talc and titanium dioxide.
Hydroxychloroquine Sulfate Tablets, USP contains 300 mg hydroxychloroquine
sulfate, equivalent to 232.5 mg base, and are for oral administration.
Inactive Ingredients: corn starch, crospovidone, hydroxypropyl
methylcellulose, lactose monohydrate, magnesium stearate, polyethylene glycol,
polyvinyl alcohol, talc, titanium dioxide and yellow iron oxide.
Hydroxychloroquine Sulfate Tablets, USP contain 400 mg hydroxychloroquine
sulfate, equivalent to 310 mg base, and are for oral administration.
Inactive Ingredients: corn starch, crospovidone, hydroxypropyl
methylcellulose, lactose monohydrate, magnesium stearate, polyethylene glycol,
polyvinyl alcohol, talc and titanium dioxide.
Meets USP Dissolution Test 1
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Important Administration Instructions
Advise the patient to take hydroxychloroquine sulfate tablets with food or milk and not to crush or divide the tablet.
Cardiomyopathy and Ventricular Arrhythmias
Inform the patient that serious cardiac effects, life-threatening and fatal cases have been reported with use of hydroxychloroquine sulfate tablets Advise patients to seek medical attention immediately if they experience any symptoms of heart rhythm changes including fast or irregular heartbeat, lightheadedness, dizziness, or syncope [see Warnings and Precautions (5.1)].
Retinal Toxicity
Inform the patient that irreversible retinal damage has been observed in some patients with the use of hydroxychloroquine sulfate tablets. Advise patients of the importance of the ophthalmology visits for monitoring their eyes. Instruct patients to seek medical attention promptly if they experience decreased vision or decreased dark adaptation [see Warnings and Precautions (5.2)].
Serious Skin Reactions
Inform the patient that severe, life-threatening skin reactions have been reported with the use of hydroxychloroquine sulfate tablets. Advise the patient to seek medical attention immediately if experiencing any of the following signs and symptoms: blisters on the skin, eyes, lips or in the mouth, itching or burning, with or without fever [see Warnings and Precautions (5.3)].
Skeletal Muscle Myopathy or Neuropathy
Inform the patient that muscle weakness and atrophy has been reported with hydroxychloroquine sulfate tablets use Advise patients to report to the physician symptoms of muscle weakness [see Warnings and Precautions (5.8)].
Neuropsychiatric Reactions Including Suicidality
Alert patients to seek medical attention immediately if they experience new or worsening depression, suicidal thoughts, or other mood changes [see Warnings and Precautions (5.9)].
Hypoglycemia
Inform the patient that hydroxychloroquine sulfate tablets has been associated with severe hypoglycemia. Advise the patient to monitor blood sugar levels if possible and to seek medical attention if experiencing any of the signs and symptoms of hypoglycemia such as sweating, shakiness, weakness, dizziness, tachycardia, nausea, blurred vision, confusion, fainting, or loss of consciousness [see Warnings and Precautions (5.10)].
Pregnancy
Inform the patient that there is a pregnancy registry that monitors pregnancy outcomes in women exposed to hydroxychloroquine sulfate tablets during pregnancy. Encourage patients to register by contacting 1-877-311-8972 [see Use in Specific Populations (8.1)].
Manufactured by:
Appco Pharma LLC
Piscataway, NJ 08854 USA
Manufactured For:
Dr. Reddy's Laboratories Inc.
Princeton, NJ 08540 USA

Revision Date: 08/2023
200266
