CARBIDOPA
Carbidopa Tablets
Approved
Approval ID
83012ccc-aba2-45bb-9326-dc30cdeb4e3b
Product Type
HUMAN PRESCRIPTION DRUG LABEL
Effective Date
Oct 13, 2022
Manufacturers
FDA
Zydus Lifesciences Limited
DUNS: 918596198
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
CARBIDOPA
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
NDC Product Code70771-1355
Application NumberANDA209910
Product Classification
M
Marketing Category
C73584
G
Generic Name
CARBIDOPA
Product Specifications
Route of AdministrationORAL
Effective DateOctober 13, 2022
FDA Product Classification
INGREDIENTS (8)
CELLULOSE, MICROCRYSTALLINEInactive
Code: OP1R32D61U
Classification: IACT
FERRIC OXIDE YELLOWInactive
Code: EX438O2MRT
Classification: IACT
CARBIDOPAActive
Quantity: 25 mg in 1 1
Code: MNX7R8C5VO
Classification: ACTIM
CROSPOVIDONEInactive
Code: 2S7830E561
Classification: IACT
FERRIC OXIDE REDInactive
Code: 1K09F3G675
Classification: IACT
HYDROXYPROPYL CELLULOSE (1600000 WAMW)Inactive
Code: RFW2ET671P
Classification: IACT
MAGNESIUM STEARATEInactive
Code: 70097M6I30
Classification: IACT
MANNITOLInactive
Code: 3OWL53L36A
Classification: IACT
Drug Labeling Information
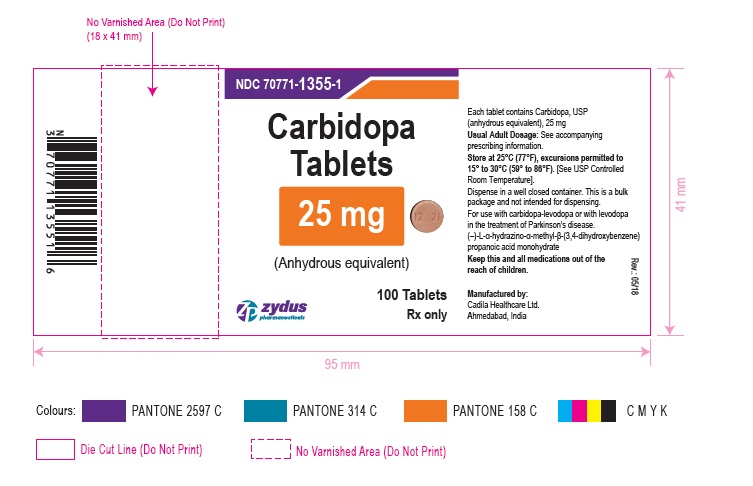
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
LOINC: 51945-4Updated: 5/25/2018
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 70771-1355-1
Carbidopa Tablets
Rx only
100 Tablets
SPL UNCLASSIFIED SECTION
LOINC: 42229-5Updated: 7/29/2020
