Felodipine
Felodipine Tablets USP
7bd4fa00-f0d8-4aea-9c24-a799b05e60e1
HUMAN PRESCRIPTION DRUG LABEL
Aug 11, 2023
Carlsbad Technology, Inc.
DUNS: 781047246
Products 3
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Felodipine
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
Felodipine
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
Felodipine
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
Drug Labeling Information
CLINICAL PHARMACOLOGY SECTION
CLINICAL PHARMACOLOGY
Mechanism of Action
Felodipine is a member of the dihydropyridine class of calcium channel
antagonists (calcium channel blockers). It reversibly competes with
nitrendipine
and/or other calcium channel blockers for dihydropyridine binding sites,
blocks voltage-dependent Ca++ currents in vascular smooth muscle and
cultured rabbit atrial cells, and blocks potassium-induced contracture of the
rat portal vein.
In vitro studies show that the effects of felodipine on contractile processes
are selective, with greater effects on vascular smooth muscle than cardiac
muscle. Negative inotropic effects can be detected in vitro, but such effects
have not been seen in intact animals.
The effect of felodipine on blood pressure is principally a consequence of a
dose related decrease of peripheral vascular resistance in man, with a modest
reflex increase in heart rate (see Cardiovascular Effects). With the exception
of a mild diuretic effect seen in several animal species and man, the effects
of felodipine are accounted for by its effects on peripheral vascular
resistance.
Pharmacokinetics and Metabolism
Following oral administration, felodipine is almost completely absorbed and
undergoes extensive first-pass metabolism. The systemic bioavailability of
felodipine extended-release tablets is approximately 20%. Mean peak
concentrations following the administration of felodipine extended-release
tablets are reached in 2.5 to 5 hours. Both peak plasma concentration and the
area under the plasma concentration time curve (AUC) increase linearly with
doses up to 20 mg. Felodipine is greater than 99% bound to plasma proteins.
Following intravenous administration, the plasma concentration of felodipine
declined triexponentially with mean disposition half-lives of 4.8 minutes,
1.5 hours, and 9.1 hours. The mean contributions of the three individual
phases to the overall AUC were 15%, 40% and 45%, respectively, in the order
of increasing t1/2.
Following oral administration of the immediate-release formulation, the plasma
level of felodipine also declined polyexponentially with a mean terminal t1/2
of
11 to 16 hours. The mean peak and trough steady-state plasma concentrations
achieved after 10 mg of the immediate-release formulation given once a day
to normal volunteers, were 20 and 0.5 nmol/L, respectively. The trough plasma
concentration of felodipine in most individuals was substantially below the
concentration needed to effect a half-maximal decline in blood pressure (EC50)
[4 to 6 nmol/L for felodipine], thus precluding once a day dosing with the
immediate-release formulation.
Following administration of a 10 mg dose of felodipine, the extended-release
formulation, to young, healthy volunteers, mean peak and trough steady-state
plasma concentrations of felodipine were 7 and 2 nmol/L, respectively.
Corresponding values in hypertensive patients (mean age 64) after a 20 mg
dose of felodipine extended-release tablets were 23 and 7 nmol/L. Since the
EC50 for felodipine is 4 to 6 nmol/L, a 5 mg to 10 mg dose of felodipine
extended-release tablets in some patients, and a 20 mg dose in others, would
be expected to provide an antihypertensive effect that persists for 24 hours
(see Cardiovascular Effects and DOSAGE AND ADMINISTRATION).
The systemic plasma clearance of felodipine in young healthy subjects is
about 0.8 L/min, and the apparent volume of distribution is about 10 L/kg.
Following an oral or intravenous dose of 14C-labeled felodipine in man, about
70% of the dose of radioactivity was recovered in urine and 10% in the feces.
A negligible amount of intact felodipine is recovered in the urine and feces
(< 0.5%). Six metabolites, which account for 23% of the oral dose, have been
identified; none has significant vasodilating activity.
Following administration of felodipine extended-release tablets to
hypertensive
patients, mean peak plasma concentrations at steady-state are about 20%
higher than after a single dose. Blood pressure response is correlated with
plasma concentrations of felodipine.
The bioavailability of felodipine extended-release tablets is influenced by
the
presence of food. When administered either with a high fat or carbohydrate
diet, Cmax is increased by approximately 60%; AUC is unchanged. When
felodipine extended-release tablets were administered after a light meal
(orange juice, toast, and cereal), however, there is no effect on felodipine's
pharmacokinetics. The bioavailability of felodipine was increased
approximately
2-fold when taken with grapefruit juice. Orange juice does not appear
to modify the kinetics of felodipine extended-release tablets. A similar
finding
has been seen with other dihydropyridine calcium antagonists, but to a lesser
extent than that seen with felodipine.
Geriatric Use
Plasma concentrations of felodipine, after a single dose and at steady-state,
increase with age. Mean clearance of felodipine in elderly hypertensives
(mean age 74) was only 45% of that of young volunteers (mean age 26).
At steady-state mean AUC for young patients was 39% of that for the elderly.
Data for intermediate age ranges suggest that the AUCs fall between the
extremes of the young and the elderly.
Hepatic Dysfunction
In patients with hepatic disease, the clearance of felodipine was reduced to
about 60% of that seen in normal young volunteers.
Renal impairment does not alter the plasma concentration profile of
felodipine;
although higher concentrations of the metabolites are present in the plasma
due to decreased urinary excretion, these are inactive.
Animal studies have demonstrated that felodipine crosses the blood-brain
barrier and the placenta.
Cardiovascular Effects
Following administration of felodipine extended-release tablets, a reduction
in blood pressure generally occurs within 2 to 5 hours. During chronic
administration, substantial blood pressure control lasts for 24 hours, with
trough reductions in diastolic blood pressure approximately 40% to 50% of
peak reductions. The antihypertensive effect is dose dependent and correlates
with the plasma concentration of felodipine.
A reflex increase in heart rate frequently occurs during the first week of
therapy; this increase attenuates over time. Heart rate increases of 5 to
10 beats per minute may be seen during chronic dosing. The increase is
inhibited by beta-blocking agents.
The P-R interval of the ECG is not affected by felodipine when administered
alone or in combination with a beta-blocking agent. Felodipine alone or in
combination with a beta-blocking agent has been shown, in clinical and
electrophysiologic studies, to have no significant effect on cardiac
conduction
(P-R, P-Q, and H-V intervals).
In clinical trials in hypertensive patients without clinical evidence of left
ventricular
dysfunction, no symptoms suggestive of a negative inotropic effect were
noted; however, none would be expected in this population (see PRECAUTIONS).
Renal/Endocrine Effects
Renal vascular resistance is decreased by felodipine while glomerular
filtration
rate remains unchanged. Mild diuresis, natriuresis, and kaliuresis have been
observed during the first week of therapy. No significant effects on serum
electrolytes were observed during short- and long-term therapy.
In clinical trials in patients with hypertension, increases in plasma
noradrenaline
levels have been observed.
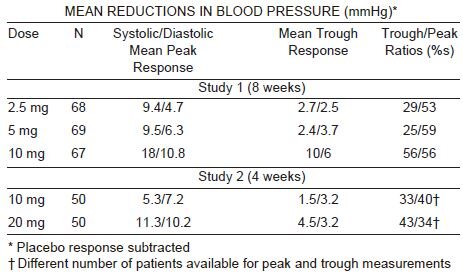
Clinical Studies
Felodipine produces dose related decreases in systolic and diastolic blood
pressure as demonstrated in six placebo-controlled, dose response studies
using either immediate-release or extended-release dosage forms. These
studies enrolled over 800 patients on active treatment, at total daily doses
ranging from 2.5 mg to 20 mg. In those studies felodipine was administered
either as monotherapy or was added to beta-blockers. The results of the two
studies with felodipine extended-release tablets given once daily as
monotherapy
are shown in the table below: