Moxifloxacin
Moxifloxacin Ophthalmic Solution
143075b4-24d4-08a1-e063-6394a90a8a6e
HUMAN PRESCRIPTION DRUG LABEL
Mar 21, 2024
Advanced Rx Pharmacy of Tennessee, LLC
DUNS: 117023142
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Moxifloxacin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL

INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
Moxifloxacin ophthalmic solution, 0.5% is indicated for the treatment of bacterial conjunctivitis caused by susceptible strains of the following organisms:
Corynebacterium species*
Micrococcus luteus*
Staphylococcus aureus
Staphylococcus epidermidis
Staphylococcus haemolyticus
Staphylococcus hominis
Staphylococcus warneri*
Streptococcus pneumoniae
Streptococcus viridans group
Acinetobacter lwoffii*
Haemophilus influenza
Haemophilus parainfluenzae*
Chlamydia trachomatis
*Efficacy for this organism was studied in fewer than 10 infections.
Moxifloxacin ophthalmic solution, 0.5% is a topical fluoroquinolone anti- infective indicated for the treatment of bacterial conjunctivitis caused by susceptible strains of the following organisms:
Corynebacterium species*, Micrococcus luteus*, Staphylococcus aureus, Staphylococcus epidermidis, Staphylococcus haemolyticus, Staphylococcus hominis, Staphylococcus warneri*, Streptococcus pneumoniae, Streptococcus viridans group, Acinetobacter lwoffii*, Haemophilus influenzae, Haemophilus parainfluenzae*, Chlamydia trachomatis
*Efficacy for this organism was studied in fewer than 10 infections. ( 1)
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
Moxifloxacin ophthalmic solution is contraindicated in patients with a history of hypersensitivity to moxifloxacin, to other quinolones, or to any of the components in this medication.
Moxifloxacin ophthalmic solution is contraindicated in patients with a history of hypersensitivity to moxifloxacin, to other quinolones, or to any of the components in this medication. ( 4)
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
In patients receiving systemically administered quinolones, including moxifloxacin, serious and occasionally fatal hypersensitivity (anaphylactic) reactions have been reported, some following the first dose. Some reactions were accompanied by cardiovascular collapse, loss of consciousness, angioedema (including laryngeal, pharyngeal or facial edema), airway obstruction, dyspnea, urticaria, and itching. If an allergic reaction to moxifloxacin occurs, discontinue use of the drug. Serious acute hypersensitivity reactions may require immediate emergency treatment. Oxygen and airway management should be administered as clinically indicated.
5.2 Growth of Resistant Organisms With Prolonged Use
As with other anti-infectives, prolonged use may result in overgrowth of non- susceptible organisms, including fungi. If superinfection occurs, discontinue use and institute alternative therapy. Whenever clinical judgment dictates, the patient should be examined with the aid of magnification, such as slit- lamp biomicroscopy, and, where appropriate, fluorescein staining.
5.3 Avoidance of Contact Lens Wear
Patients should be advised not to wear contact lenses if they have signs or symptoms of bacterial conjunctivitis.
- Hypersensitivity Reactions: Hypersensitivity and anaphylaxis have been reported with systemic use of moxifloxacin. ( 5.1)
- Prolonged Use: May result in overgrowth of non- susceptible organisms, including fungi. If superinfection occurs, discontinue use and institute alternative therapy. ( 5.2)
- Avoid Contact Lens Wear: Patients should not wear contact lenses if they have signs or symptoms of bacterial conjunctivitis. ( 5.3)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to the rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The most frequently reported ocular adverse events were conjunctivitis, decreased visual acuity, dry eye, keratitis, ocular discomfort, ocular hyperemia, ocular pain, ocular pruritus, subconjunctival hemorrhage, and tearing. These events occurred in approximately 1% to 6% of patients.
Nonocular adverse events reported at a rate of 1% to 4% were fever, increased cough, infection, otitis media, pharyngitis, rash, and rhinitis.
The most frequently reported ocular adverse events were conjunctivitis, decreased visual acuity, dry eye, keratitis, ocular discomfort, ocular hyperemia, ocular pain, ocular pruritus, subconjunctival hemorrhage, and tearing. These events occurred in approximately 1% to 6% of patients. ( 6)
To report SUSPECTED ADVERSE REACTIONS, contact Lupin Pharmaceuticals, Inc. at 1-800-399-2561 or FDA at 1-800-FDA-1088 orwww.fda.gov/medwatch.
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
Drug-drug interaction studies have not been conducted with moxifloxacin ophthalmic solution. In vitrostudies indicate that moxifloxacin does not inhibit CYP3A4, CYP2D6, CYP2C9, CYP2C19, or CYP1A2, indicating that moxifloxacin is unlikely to alter the pharmacokinetics of drugs metabolized by these cytochrome P450 isozymes.
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate and well-controlled studies with moxifloxacin ophthalmic solution in pregnant women to inform any drug-associated risks.
Oral administration of moxifloxacin to pregnant rats and monkeys and intravenously to pregnant rabbits during the period of organogenesis did not produce adverse maternal or fetal effects at clinically relevant doses. Oral administration of moxifloxacin to pregnant rats during late gestation through lactation did not produce adverse maternal, fetal or neonatal effects at clinically relevant doses (see DATA).
Data
Animal Data
Embryo-fetal studies were conducted in pregnant rats administered with 20, 100, or 500 mg/kg/day moxifloxacin by oral gavage on Gestation Days 6 to 17, to target the period of organogenesis. Decreased fetal body weight and delayed skeletal development were observed at 500 mg/kg/day [277 times the human area under the curve (AUC) at the recommended human ophthalmic dose]. The No- Observed-Adverse-Effect-Level (NOAEL) for developmental toxicity was 100 mg/kg/day (30 times the human AUC at the recommended human ophthalmic dose).
Embryo-fetal studies were conducted in pregnant rabbits administered with 2, 6.5, or 20 mg/kg/day moxifloxacin by intravenous administration on Gestation Days 6 to 20, to target the period of organogenesis. Abortions, increased incidence of fetal malformations, delayed fetal skeletal ossification, and reduced placental and fetal body weights were observed at 20 mg/kg/day (1086 times the human AUC at the recommended human ophthalmic dose), a dose that produced maternal body weight loss and death. The NOAEL for developmental toxicity was 6.5 mg/kg/day (246 times the human AUC at the recommended human ophthalmic dose).
Pregnant cynomolgus monkeys were administered moxifloxacin at doses of 10, 30, or 100 mg/kg/day by intragastric intubation between Gestation Days 20 and 50, targeting the period of organogenesis. At the maternal toxic doses of ≥ 30 mg/kg/day, increased abortion, vomiting, and diarrhea were observed. Smaller fetuses/reduced fetal body weights were observed at 100 mg/kg/day (2864 times the human AUC at the recommended human ophthalmic dose). The NOAEL for fetal toxicity was 10 mg/kg/day (174 times the human AUC at the recommended human ophthalmic dose).
In a pre- and postnatal study, rats were administered moxifloxacin by oral gavage at doses of 20, 100, and 500 mg/kg/day from Gestation Day 6 until the end of lactation. Maternal death occurred during gestation at 500 mg/kg/day. Slight increases in the duration of pregnancy, reduced pup birth weight, and decreased prenatal and neonatal survival were observed at 500 mg/kg/day (estimated 277 times the human AUC at the recommended human ophthalmic dose). The NOAEL for pre- and postnatal development was 100 mg/kg/day (estimated 30 times the human AUC at the recommended human ophthalmic dose).
8.2 Lactation
Risk Summary
There is no data regarding the presence of moxifloxacin in human milk, the effects on the breastfed infants, or the effects on milk production/excretion to inform risk of moxifloxacin ophthalmic solution to an infant during lactation.
A study in lactating rats has shown transfer of moxifloxacin into milk following oral administration.
Systemic levels of moxifloxacin following topical ocular administration are low [see CLINICAL PHARMACOLOGY ( 12.3)] , and it is not known whether measurable levels of moxifloxacin would be present in maternal milk following topical ocular administration.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for moxifloxacin ophthalmic solution and any potential adverse effects on the breastfed child from moxifloxacin ophthalmic solution .
8.4 Pediatric Use
The safety and effectiveness of moxifloxacin ophthalmic solution have been established in all ages. Use of moxifloxacin ophthalmic solution is supported by evidence from adequate and well controlled studies of moxifloxacin ophthalmic solution in adults, children, and neonates [see CLINICAL STUDIES ( 14)] .
There is no evidence that the ophthalmic administration of moxifloxacin ophthalmic solution has any effect on weight bearing joints, even though oral administration of some quinolones has been shown to cause arthropathy in immature animals.
8.5 Geriatric Use
No overall differences in safety and effectiveness have been observed between elderly and younger patients.
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
Instill one drop in the affected eye 3 times a day for 7 days. Moxifloxacin ophthalmic solution is for topical ophthalmic use.
Instill one drop in the affected eye 3 times a day for 7 days. ( 2)
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
Ophthalmic solution containing moxifloxacin 0.5% USP.
Ophthalmic solution containing moxifloxacin 0.5%. ( 3)
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Moxifloxacin is a member of the fluoroquinolone class of anti-infective drugs [see MICROBIOLOGY ( 12.4)] .
12.3 Pharmacokinetics
Plasma concentrations of moxifloxacin were measured in healthy adult male and female subjects who received bilateral topical ocular doses of moxifloxacin ophthalmic solution 3 times a day.
The mean steady-state C max(2.7 ng/mL) and AUC 0 to ∞(41.9 ng•hr/mL) values were 1600 and 1100 times lower than the mean C maxand AUC reported after therapeutic 400 mg doses of moxifloxacin. The plasma half-life of moxifloxacin was estimated to be 13 hours.
12.4 Microbiology
The antibacterial action of moxifloxacin results from inhibition of the topoisomerase II (DNA gyrase) and topoisomerase IV. DNA gyrase is an essential enzyme that is involved in the replication, transcription and repair of bacterial DNA. Topoisomerase IV is an enzyme known to play a key role in the partitioning of the chromosomal DNA during bacterial cell division.
The mechanism of action for quinolones, including moxifloxacin, is different from that of macrolides, aminoglycosides, or tetracyclines. Therefore, moxifloxacin may be active against pathogens that are resistant to these antibiotics and these antibiotics may be active against pathogens that are resistant to moxifloxacin. There is no cross-resistance between moxifloxacin and the aforementioned classes of antibiotics. Cross-resistance has been observed between systemic moxifloxacin and some other quinolones.
In vitro resistance to moxifloxacin develops via multiple-step mutations. Resistance to moxifloxacin occurs in vitro at a general frequency of between 1.8 x 10 -9to less than 1 x 10 -11for gram-positive bacteria.
Moxifloxacin has been shown to be active against most strains of the following microorganisms, both in vitro and in clinical infections as described in the Indications and Usage section:
Aerobic Gram-Positive Microorganisms
Corynebacterium species*
Micrococcus luteus*
Staphylococcus aureus
Staphylococcus epidermidis
Staphylococcus haemolyticus
Staphylococcus hominis
Staphylococcus warneri*
Streptococcus pneumoniae
Streptococcus viridans group
Aerobic Gram-Negative Microorganisms
Acinetobacter lwoffii*
Haemophilus influenza
Haemophilus parainfluenzae*
Other Microorganisms
Chlamydia trachomatis
*Efficacy for this organism was studied in fewer than 10 infections.
The following in vitro data are also available, but their clinical significance in ophthalmic infections is unknown. The safety and effectiveness of moxifloxacin ophthalmic solution in treating ophthalmological infections due to these microorganisms have not been established in adequate and well- controlled trials.
The following organisms are considered susceptible when evaluated using systemic breakpoints. However, a correlation between the in vitro systemic breakpoint and ophthalmological efficacy has not been established. The list of organisms is provided as guidance only in assessing the potential treatment of conjunctival infections. Moxifloxacin exhibits in vitro minimal inhibitory concentrations (MICs) of 2 microgram/mL or less (systemic susceptible breakpoint) against most (greater than or equal to 90%) strains of the following ocular pathogens.
Aerobic Gram-Positive Microorganisms
Listeria monocytogenes
Staphylococcus saprophyticus
Streptococcus agalactiae
Streptococcus mitis
Streptococcus pyogenes
Streptococcus Group C, G, and F
Aerobic Gram-Negative Microorganisms
Acinetobacter baumannii
Acinetobacter calcoaceticus
Citrobacter freundii
Citrobacter koseri
Enterobacter aerogenes
Enterobacter cloacae
Escherichia coli
Klebsiella oxytoca
Klebsiella pneumoniae
Moraxella catarrhalis
Morganella morganii
Neisseria gonorrhoeae
Proteus mirabilis
Proteus vulgaris
Pseudomonas stutzeri
Anaerobic Microorganisms
Clostridium perfringens
Fusobacterium species
Prevotella species
Propionibacterium acnes
Other Microorganisms
Chlamydia pneumoniae
Legionella pneumophila
Mycobacterium avium
Mycobacterium marinum
Mycoplasma pneumoniae
DESCRIPTION SECTION
11 DESCRIPTION
Moxifloxacin ophthalmic solution USP, 0.5% is a sterile solution for topical ophthalmic use. Moxifloxacin hydrochloride is an 8-methoxy fluoroquinolone anti-infective, with a diazabicyclononyl ring at the C7 position. The chemical name for moxifloxacin hydrochloride is 1-Cyclopropyl-6-fluoro-1,4-dihydro-8-methoxy-7-[(4aS,7aS)-octahydro-6H-pyrrolol[3,4- b]pyridin-6-yl]-4-oxo-3-quinolinecarboxylic acid, monohydrochloride. The molecular formula for moxifloxacin hydrochloride is C 21H 24FN 3O 4•HCl and its molecular weight is 437.9 g/mol.
The chemical structure is presented below:
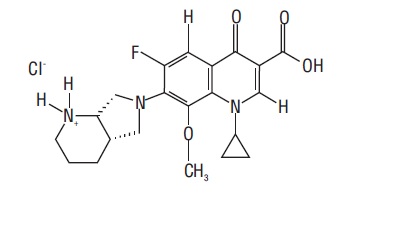
Moxifloxacin hydrochloride USP is a slightly yellow to yellow crystalline powder.
Each mL of moxifloxacin ophthalmic solution USP contains 5.45 mg moxifloxacin hydrochloride USP equivalent to 5 mg moxifloxacin base.
Moxifloxacin ophthalmic solution contains:
**Active:**Moxifloxacin 0.5% (5 mg/mL);
**Inactives:**Boric acid, sodium chloride and water for injection. May also contain hydrochloric acid and/or sodium hydroxide to adjust pH to approximately 6.8.
Moxifloxacin ophthalmic solution USP, 0.5% is a yellow colored transparent isotonic solution with an osmolality of approximately 290 mOsm/kg.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
In two randomized, double-masked, multicenter, controlled clinical trials in which patients were dosed 3 times a day for 4 days, moxifloxacin ophthalmic solution produced clinical cures on Day 5 to 6 in 66% to 69% of patients treated for bacterial conjunctivitis. Microbiological success rates for the eradication of baseline pathogens ranged from 84% to 94%.
In a randomized, double-masked, multicenter, parallel-group clinical trial of pediatric patients with bacterial conjunctivitis between birth and 31 days of age, patients were dosed with moxifloxacin ophthalmic solution or another anti-infective agent. Clinical outcomes for the trial demonstrated a clinical cure rate of 80% at Day 9 and a microbiological eradication success rate of 92% at Day 9.
Please note that microbiologic eradication does not always correlate with clinical outcome in anti-infective trials.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Moxifloxacin ophthalmic solution USP, 0.5% is supplied as a sterile ophthalmic solution in a sterile 5 mL natural low density polyethylene bottle fitted with a natural low density polyethylene nozzle and sealed with tan colored high density polyethylene cap as follows:
3 mL in 5 mL bottle NDC: 80425-0379-01
Storage: Store at 2°C to 25°C (36°F to 77°F).
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term studies in animals to determine the carcinogenic potential of moxifloxacin have not been performed. However, in an accelerated study with initiators and promoters, moxifloxacin was not carcinogenic in rats following up to 38 weeks of oral dosing at 500 mg/kg/day (3224 times the highest recommended total daily human ophthalmic dose for a 60 kg person, based on body surface area).
Mutagenesis
Moxifloxacin was not mutagenic in four bacterial strains used in the Ames Salmonellareversion assay. As with other quinolones, the positive response observed with moxifloxacin in strain TA 102 using the same assay may be due to the inhibition of DNA gyrase. Moxifloxacin was not mutagenic in the CHO/HGPRT mammalian cell gene mutation assay. An equivocal result was obtained in the same assay when V79 cells were used. Moxifloxacin was clastogenic in the V79 chromosome aberration assay, but it did not induce unscheduled DNA synthesis in cultured rat hepatocytes. There was no evidence of genotoxicity in vivoin a micronucleus test or a dominant lethal test in mice.
Impairment of Fertility
Moxifloxacin had no effect on fertility in male and female rats at oral doses as high as 500 mg/kg/day, approximately 3224 times the highest recommended total daily human ophthalmic dose, based on body surface area. At 500 mg/kg/day orally there were slight effects on sperm morphology (head-tail separation) in male rats and on the estrous cycle in female rats.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Avoid Contamination of the Product
Advise patients not to touch the dropper tip to any surface to avoid contaminating the contents.
Avoid Contact Lens Wear
Advise patients not to wear contact lenses if they have signs and symptoms of bacterial conjunctivitis [see WARNINGS AND PRECAUTIONS ( 5.3) ].
Hypersensitivity Reactions
Systemically administered quinolones including moxifloxacin have been associated with hypersensitivity reactions, even following a single dose. Instruct patients to discontinue use immediately and contact their physician at the first sign of a rash or allergic reaction [see WARNINGS AND PRECAUTIONS ( 5.1)].
Rx Only
Manufactured for:
Lupin Pharmaceuticals, Inc.
Baltimore, Maryland 21202
United States.
Manufactured by:
Lupin Limited
Pithampur (M. P.), 454 775
India.
June 2022 ID #: 269219
Distributed by:
Advanced Rx Pharmacy of Tennessee, LLC
