Allergy Relief
walgreens-Diphenhydramine HCl Capsules, 50 mg dye free
2969ba8f-c477-222a-e063-6394a90af703
HUMAN OTC DRUG LABEL
May 27, 2025
WALGREEN COMPANY
DUNS: 008965063
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Diphenhydramine HCl
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
Drug Labeling Information
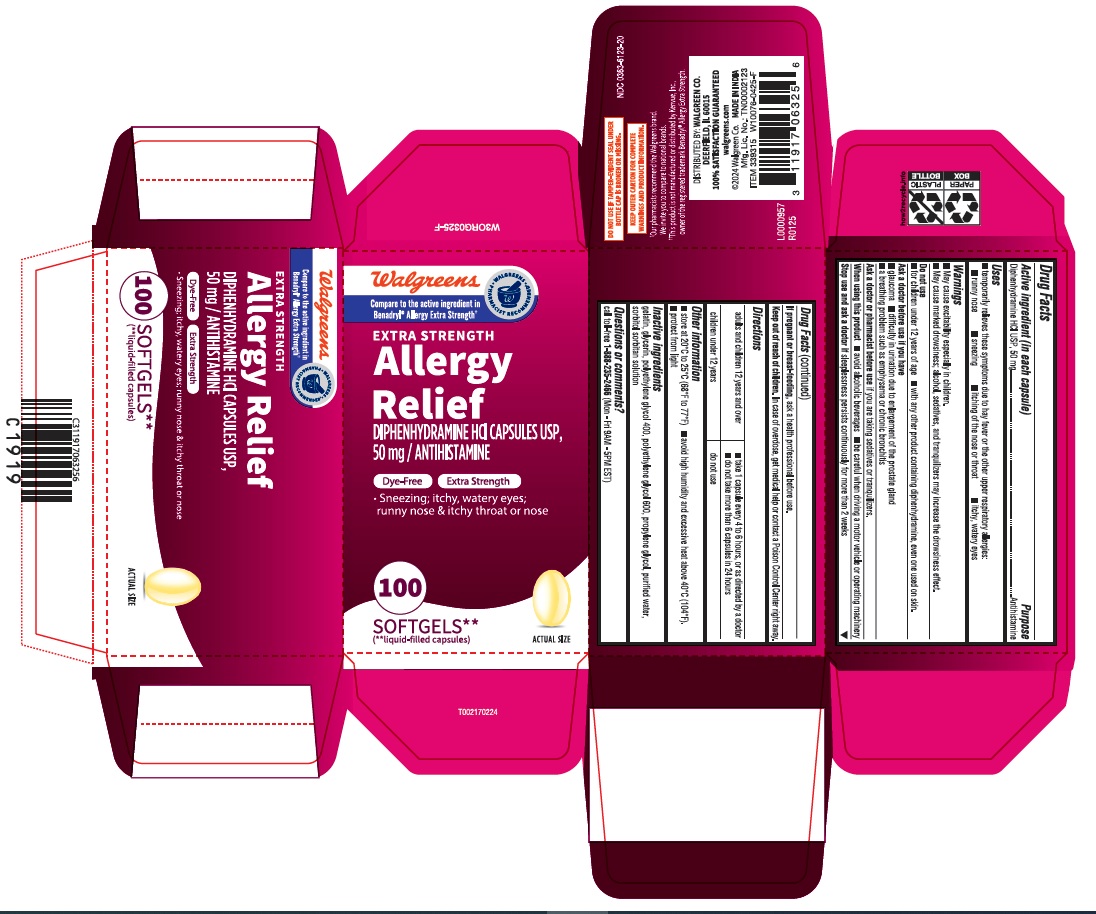
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
carton
Walgreens
Compare to the active ingredient in
Benadryl® Allergy Extra Strength
EXTRA STRENGTH
Allergy
Relief
DIPHENHYDRAMINE HCl CAPSULES USP,
50 mg/ANTIHISTAMINE
Dye-Free Extra Strength
Sneezing, itchy, watery eyes;
runny nose & itchy throat or nose
100
SOFTGELS**
(** liquid-filled capsules)
INDICATIONS & USAGE SECTION
Uses
•temporarily relieves these symptoms due to hay fever or the other upper
respiratory allergies:
• runny nose • sneezing • itching of the nose or throat • itchy, watery eyes
OTC - ASK DOCTOR/PHARMACIST SECTION
Ask a doctor or pharmacist before use
if you are taking sedatives or tranquilizers.
OTC - WHEN USING SECTION
When using this product
■ avoid alcoholic beverages ■ be careful when driving a motor vehicle or operating machinery
OTC - STOP USE SECTION
Stop use and ask a doctor if
sleeplessness persists continuosly for more than 2 weeks
OTC - PREGNANCY OR BREAST FEEDING SECTION
If pregnant or breast- feeding,
ask a health professional before use.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
OTC - ACTIVE INGREDIENT SECTION
Active ingredient (in each capsule)
Diphenhydramine HCl 50 mg
OTC - PURPOSE SECTION
Purpose
Antihistamine
WARNINGS SECTION
Warnings
• May cause excitability especially in children.
• May cause marked drowsiness; alcohol, sedatives, and tranquilizers may
increase the drowsiness effect.
OTC - DO NOT USE SECTION
Do not use
• for children under 12 years of age • with any other product conaining diphenhydramine, even one used on skin.
OTC - ASK DOCTOR SECTION
Ask a doctor before use if you have
■ glaucoma ■ difficulty in urination due to enlargement of the prostate gland
■ a breathing problem such as emphysema or chronic bronchitis
DOSAGE & ADMINISTRATION SECTION
Directions
|
adults and children 12 years and over |
• take 1 capsule every 4 to 6 hours, or as directed by a doctor |
|
children under 12 years |
do not use |
STORAGE AND HANDLING SECTION
Other information
■ store at 20°C to 25°C (68°F to 77°F) ■ avoid high humidity and excessive
heat above 40°C (104°F).
■ protect from light
INACTIVE INGREDIENT SECTION
Inactive ingredients
gelatin, glycerin, polyethylene glycol 400, polyethylene glycol 600, propylene glycol, purified water, sorbitol sorbitan solution
OTC - QUESTIONS SECTION
Questions or comments?
call toll-free1-888-235-2466 (Mon - Fri 9AM - 5PM EST)
SPL UNCLASSIFIED SECTION
DO NOT USE IF TAMPER-EVIDENT SEAL UNDER BOTTLE CAP IS BROKEN OR MISSING.
** KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION**
ƚOur pharmacists recommend the Walgreens brand.
We invite you to compare to national brands.
ƚƚThis product is not manufactured or distributed by Kenvue, Inc.,
owner of the registered trademark Benadryl ® Allergy Extra Strength.
DISTRIBUTED BY: WALGREENS CO.
DEERFIELD, IL 60015
100% SATISFACTION GUARANTEED
wlagreens.com
©2024 Walgreen Co,MADE IN INDIA
Mfg. Lic. No.: TN00002123
L0000957
R0125
Lot No.:
Exp. Date:
