TEZSPIRE
These highlights do not include all the information needed to use TEZSPIRE safely and effectively. See full prescribing information for TEZSPIRE. TEZSPIRE (tezepelumab-ekko) injection, for subcutaneous useInitial U.S. Approval: 2021
60f0aa03-ad25-4d48-80ce-7fcfa76f325f
HUMAN PRESCRIPTION DRUG LABEL
May 26, 2023
Amgen Inc
DUNS: 039976196
Amgen, Inc
DUNS: 039976196
Products 3
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
tezepelumab-ekko
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
tezepelumab-ekko
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
tezepelumab-ekko
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC 55513-123-01 Rx only
TEZSPIRE®
(tezepelumab-ekko)
Injection
210 mg/1.91 mL (110 mg/mL)
For Subcutaneous Injection Only
Store the pre-filled pen refrigerated at 36°F to 46°F
(2°C to 8°C) in original carton to protect from light.
DO NOT SHAKE FREEZE OR EXPOSE TO HEAT.
1 Single-dose pre-filled pen.
Discard unused portion.
ATTENTION: Follow enclosed “Instructions for Use”
to prepare and deliver your dose.
AMGEN® AstraZeneca

DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The recommended dosage of TEZSPIRE is 210 mg administered subcutaneously once every 4 weeks.
Missed Dose Information
If a dose is missed, administer the dose as soon as possible. Thereafter, the patient can continue (resume) dosing on the usual day of administration. If the next dose is already due, then administer as planned.
2.2 Preparation and Administration Instructions
TEZSPIRE vial and pre‑filled syringe are intended for administration by a healthcare provider.
TEZSPIRE pre-filled pen can be administered by patients/caregivers or healthcare providers. Patients/caregivers may administer TEZSPIRE pre-filled pen after proper training in subcutaneous injection technique and after the healthcare provider determines it is appropriate.
Each vial, pre-filled syringe and pre‑filled pen contain a single dose of TEZSPIRE.
•
Prior to administration, remove TEZSPIRE from the refrigerator and allow it to reach room temperature. This generally takes 60 minutes. Do not expose to heat and do not shake. Do not use if the security seal on the carton has been broken. Do not put back in the refrigerator once TEZSPIRE has reached room temperature.
•
Visually inspect TEZSPIRE for particulate matter and discoloration prior to administration. TEZSPIRE is a clear to opalescent, colorless to light yellow solution. Do not use TEZSPIRE if liquid is cloudy, discolored, or if it contains large particles or foreign particulate matter. Do not use if the vial, pre-filled syringe or pre‑filled pen has been dropped or damaged or if the expiration date has passed.
•
Inject TEZSPIRE 210 mg (contents of one vial, one pre-filled syringe or one pre-filled pen as described below) subcutaneously into the thigh or abdomen, except for the 2 inches (5 cm) around the navel. If a healthcare provider or caregiver administers the injection, the upper arm can also be used. A patient should not self-inject in the upper arm. TEZSPIRE should not be injected into areas where the skin is tender, bruised, erythematous, or hardened. It is recommended to rotate the injection site with each injection.
Administration Instructions for Single-Dose Pre-filled Syringe
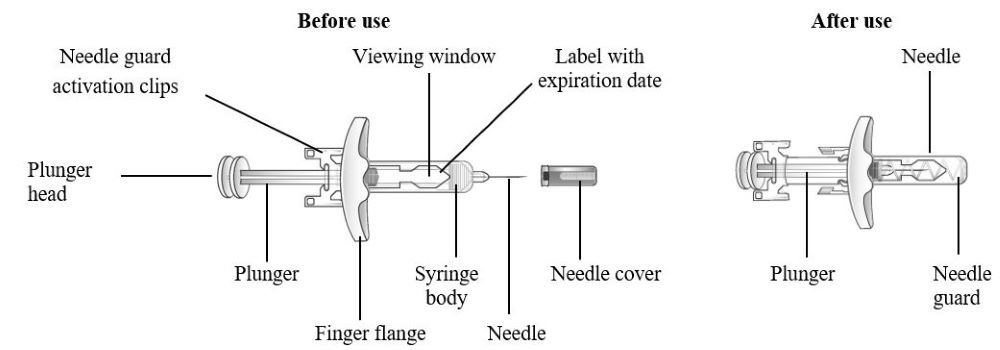
Refer to Figure 1 to identify the pre-filled syringe components for use in the administration steps.
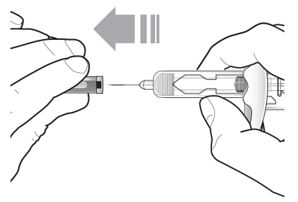
Do not remove the needle cover until Step 2 of these instructions when you are ready to inject TEZSPIRE. Do not touch the needle guard activation clips to prevent premature activation of the needle safety guard.
Figure 1 TEZSPIRE Pre-filled Syringe Components
|
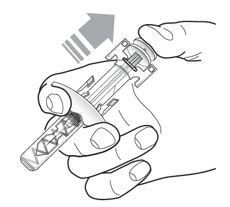
Grasp the syringe body to remove the pre-filled syringe from its tray. Do not grab the pre-filled syringe by the plunger. The pre-filled syringe may contain small air bubbles; this is normal. Do not expel the air bubbles prior to administration. | |
|
|
Do not remove the needle cover until ready to inject. Hold the syringe body and remove the needle cover by pulling straight off. Do not hold the plunger or plunger head while removing the needle cover. You may see a drop of liquid at the end of the needle. This is normal. |
|
|
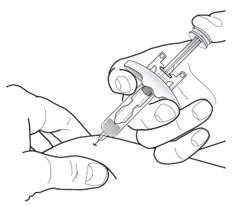
Gently pinch the skin and administer subcutaneously at approximately 45° angle into the recommended injection site (i.e., upper arm, thigh, or abdomen). |
|
|
Inject all of the medication by pushing in the plunger all the way until the plunger head is completely between the needle guard activation clips. This is necessary to activate the needle guard. |
|
|
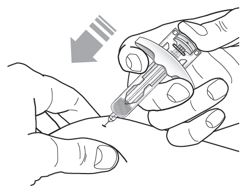
After injection, maintain pressure on the plunger head and remove the needle from the skin. Release pressure on the plunger head to allow the needle guard to cover the needle. Do not re-cap the pre-filled syringe. |
|
Discard the used syringe into a sharps container. |
Administration Instructions for Single-Dose Pre-filled Pen
These administration instructions are intended for healthcare providers use only. Patients and caregivers should refer to the TEZSPIRE pre-filled pen ‘Instructions for Use’ for more detailed instructions on the preparation and administration of TEZSPIRE pre-filled pen [See Instructions for Use].
Patients/caregivers may inject after proper training in subcutaneous injection technique according to the ‘Instructions for Use’, and after the healthcare provider determines it is appropriate.
Refer to Figure 2 to identify the pre-filled pen components for use in the administration steps.
Do not remove the cap until you are ready to inject TEZSPIRE.
Figure 2 TEZSPIRE Pre-filled Pen Components

|
Grab the middle of the pre-filled pen body to remove the pre-filled pen from its tray. The pre-filled pen may contain small air bubbles; this is normal. Do not expel the air bubbles prior to administration. | |
|
|
Do not remove the cap until ready to inject. Hold the pre-filled pen body with 1 hand and carefully pull the cap straight off with your other hand. Do not touch the needle or push the orange needle guard with your finger. Do not put the cap back on the pre-filled pen. You could cause the injection to happen too soon or damage the needle. |
|
Gently pinch the skin at the injection site or give the injection without pinching the skin. Inject TEZSPIRE by following the steps into the recommended injection site (i.e., upper arm, thigh, or abdomen). When injecting, you will hear the first click that tells you the injection has started. Press and hold the pre-filled pen for 15 seconds until you hear the second click. Do not change the position of the pre-filled pen after the injection has started. | |
|
|
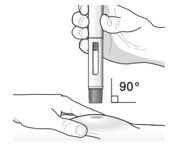
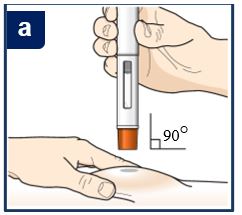
Position the pre-filled pen. Place the orange needle guard flat against the skin (90‑degree angle). Make sure you can see the viewing window. |
|
|
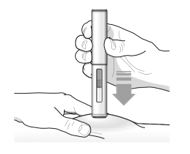
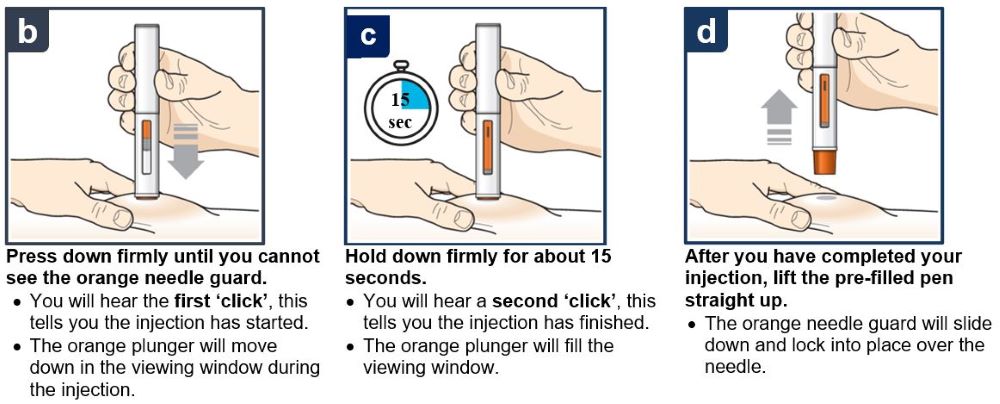
Press down firmly until you cannot see the orange needle guard. You will hear the first ‘click’, this tells you the injection has started. The orange plunger will move down in the viewing window during the injection. |
|
|
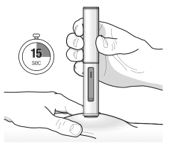
Hold down firmly for about 15 seconds. You will hear a second ‘click’, this tells you the injection has finished. The orange plunger will fill the viewing window. |
|
|
After you have completed the injection, lift the pre-filled pen straight up. The orange needle guard will slide down and lock into place over the needle. |
|
Discard the used pre-filled pen into a sharps container. |
•
Administer by subcutaneous injection. (2.1)
•
Recommended dosage is 210 mg administered once every 4 weeks. (2.1)
•
See full prescribing information for preparation and administration instructions. (2.2)
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
The efficacy of TEZSPIRE was evaluated in two randomized, double-blind, parallel group, placebo-controlled clinical trials (PATHWAY [NCT02054130] and NAVIGATOR [NCT03347279]) of 52 weeks duration. The two trials enrolled a total of 1609 patients 12 years of age and older with severe asthma.
PATHWAY was a 52-week dose-ranging exacerbation trial that enrolled 550 adult patients with severe asthma who received treatment with tezepelumab-ekko 70 mg subcutaneously every 4 weeks, TEZSPIRE 210 mg subcutaneously every 4 weeks, tezepelumab-ekko 280 mg subcutaneously every 2 weeks, or placebo subcutaneously. Patients were required to have a history of 2 or more asthma exacerbations requiring oral or injectable corticosteroid treatment or 1 asthma exacerbation resulting in hospitalization in the past 12 months.
NAVIGATOR was a 52-week exacerbation trial that enrolled 1061 patients (adult and pediatric patients 12 years of age and older) with severe asthma who received treatment with TEZSPIRE 210 mg subcutaneously every 4 weeks or placebo subcutaneously every 4 weeks. Patients were required to have a history of 2 or more asthma exacerbations requiring oral or injectable corticosteroid treatment or resulting in hospitalization in the past 12 months.
In both PATHWAY and NAVIGATOR, patients were required to have an Asthma Control Questionnaire 6 (ACQ-6) score of 1.5 or more at screening and reduced lung function at baseline [pre-bronchodilator forced expiratory volume in 1 second (FEV1) below 80% predicted in adults, and below 90% predicted in adolescents]. Patients were required to have been on regular treatment with medium or high-dose inhaled corticosteroids (ICS) and at least one additional asthma controller, with or without oral corticosteroids (OCS). Patients continued background asthma therapy throughout the duration of the trials. In both trials, patients were enrolled without requiring a minimum baseline level of blood eosinophils or FeNO.
The demographics and baseline characteristics of PATHWAY and NAVIGATOR are provided in Table 2 below.
Table 2 Demographics and Baseline Characteristics of Patients in PATHWAY and NAVIGATOR|
PATHWAY |
NAVIGATOR | |
|---|---|---|
| ||
|
Mean age (year) (SD) |
52 (12) |
50 (16) |
|
Female (%) |
66 |
64 |
|
White (%) |
92 |
62 |
|
Black or African American (%) |
3 |
6 |
|
Asian (%) |
3 |
28 |
|
Hispanic or Latino (%) |
1 |
15 |
|
Never smoked (%) |
81 |
80 |
|
High-dose ICS use (%) |
49 |
75 |
|
OCS use (%) |
9 |
9 |
|
Mean number of exacerbations in previous year (SD) |
2.4 (1.2) |
2.8 (1.4) |
|
Mean duration of asthma (years) (SD) |
17 (12) |
22 (16) |
|
Mean baseline % predicted FEV1 (SD) |
60 (13) |
63 (18) |
|
Mean post-bronchodilator FEV1 reversibility (%) (SD) |
23 (20) |
15 (15) |
|
Mean baseline blood EOS count (cells/µL) (SD) |
371 (353) |
340 (403) |
|
Positive serum specific IgE to any perennial allergen (%)* |
46 |
64 |
|
Mean FeNO (ppb) (SD) |
35 (39) |
44 (41) |
EOS, Eosinophils; FEIA, Fluorescent enzyme immunoassay; FeNO, Fractional exhaled nitric oxide; FEV1, Forced expiratory volume in one second; ICS, Inhaled corticosteroid, IgE, Immunoglobulin E; OCS, Oral corticosteroid; ppb, Parts per billion; SD, Standard deviation.
The results summarized below are for the recommended TEZSPIRE 210 mg subcutaneously every 4 weeks dosing regimen.
Exacerbations
The primary endpoint for PATHWAY and NAVIGATOR was the rate of clinically significant asthma exacerbations measured over 52 weeks. Clinically significant asthma exacerbations were defined as worsening of asthma requiring the use of or increase in oral or injectable corticosteroids for at least 3 days, or a single depo-injection of corticosteroids, and/or emergency department visits requiring use of oral or injectable corticosteroids and/or hospitalization.
In both PATHWAY and NAVIGATOR, patients receiving TEZSPIRE had significant reductions in the annualized rate of asthma exacerbations compared to placebo. There were also fewer exacerbations requiring emergency room visits and/or hospitalization in patients treated with TEZSPIRE compared with placebo (Table 3).
Table 3 Rate of Clinically Significant Exacerbations Over 52 Weeks in PATHWAY and NAVIGATOR|
Trial |
Treatment |
Exacerbations per year | |
|---|---|---|---|
|
Rate |
Rate Ratio (95% CI) | ||
|
Annualized Asthma Exacerbation Rate | |||
|
PATHWAY |
TEZSPIRE (N=137) |
0.20 |
0.29 (0.16, 0.51) |
|
Placebo (N=138) |
0.72 | ||
|
NAVIGATOR |
TEZSPIRE (N=528) |
0.93 |
0.44 (0.37, 0.53) |
|
Placebo (N=531) |
2.10 | ||
|
Exacerbations requiring emergency room visit/hospitalization | |||
|
PATHWAY |
TEZSPIRE (N=137) |
0.03 |
0.15 (0.04, 0.58) |
|
Placebo (N=138) |
0.18 | ||
|
NAVIGATOR |
TEZSPIRE (N=528) |
0.06 |
0.21 (0.12, 0.37) |
|
Placebo (N=531) |
0.28 | ||
|
Exacerbations requiring hospitalization | |||
|
PATHWAY |
TEZSPIRE (N=137) |
0.02 |
0.14 (0.03, 0.71) |
|
Placebo (N=138) |
0.14 | ||
|
NAVIGATOR |
TEZSPIRE (N=528) |
0.03 |
0.15 (0.07, 0.22) |
|
Placebo (N=531) |
0.19 |
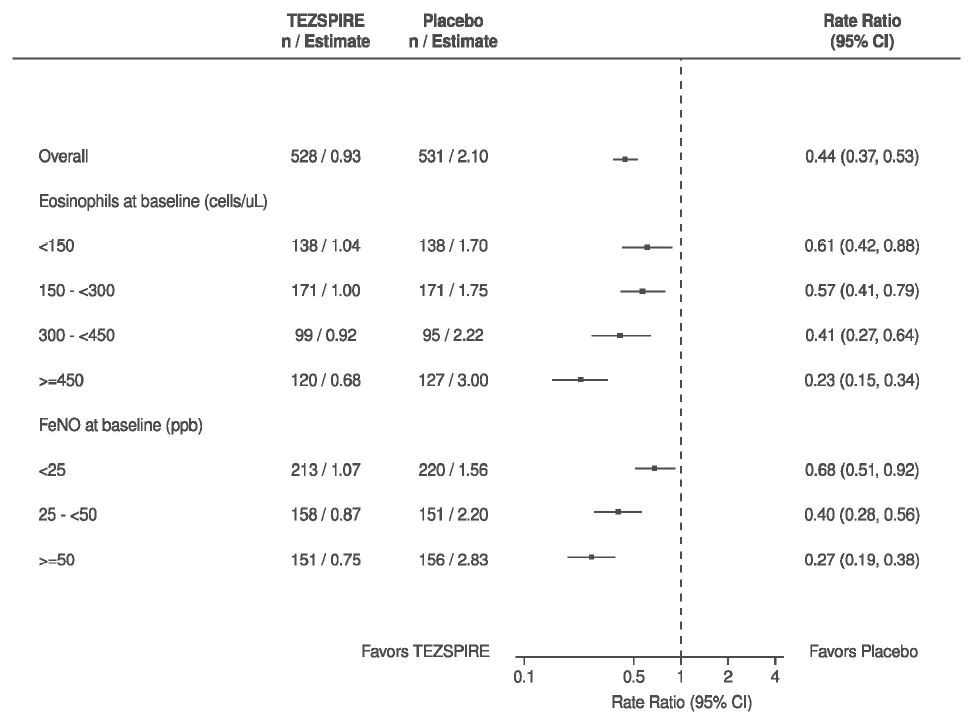
In NAVIGATOR, patients receiving TEZSPIRE experienced fewer exacerbations than those receiving placebo regardless of baseline levels of blood eosinophils or FeNO (Figure 3). Similar results were seen in PATHWAY.
Figure 3 Annualized Asthma Exacerbation Rate Ratio Over 52 Weeks Across Different Baseline Biomarkers in NAVIGATOR
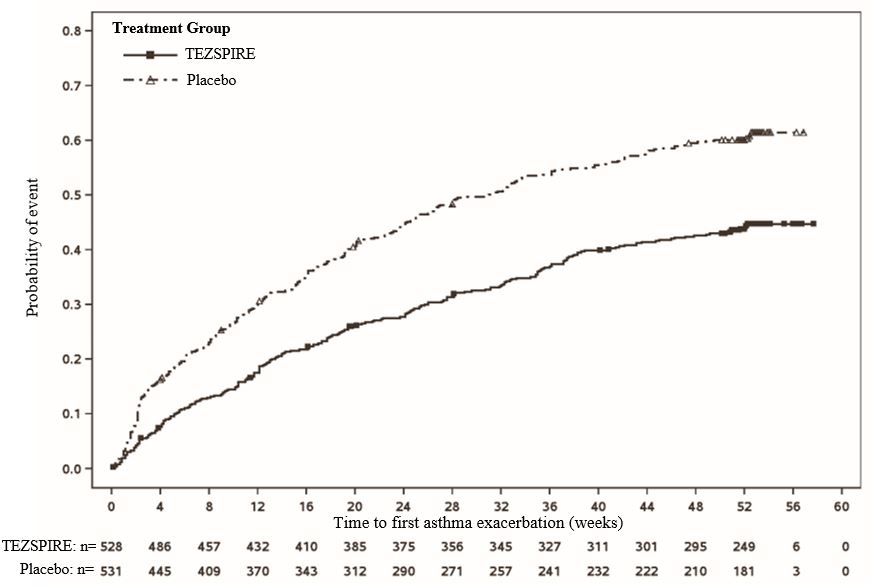
The time to first exacerbation was longer for the patients receiving TEZSPIRE compared with placebo in NAVIGATOR (Figure 4). Similar findings were seen in PATHWAY.
**Figure 4 Kaplan-Meier Cumulative Incidence Curves for Time to First
Exacerbation in NAVIGATOR
**
Lung Function
Change from baseline in FEV1 was assessed as a secondary endpoint in PATHWAY and NAVIGATOR. Compared with placebo, TEZSPIRE provided clinically meaningful improvements in the mean change from baseline in FEV1 in both trials (Table 4).
Table 4 Mean Change from Baseline in Pre-Bronchodilator FEV1 at End of Trial in PATHWAY and NAVIGATOR*
| |||
|
Trial |
Treatment |
LS Mean Change from Baseline (L) |
Difference from Placebo (95% CI) |
|
PATHWAY |
TEZSPIRE (N=133)† |
0.08 |
0.13 (0.03, 0.23) |
|
Placebo (N=138)† |
-0.06 | ||
|
NAVIGATOR |
TEZSPIRE (N=527)† |
0.23 |
0.13 (0.08, 0.18) |
|
Placebo (N=531)† |
0.10 |
In NAVIGATOR, improvement in FEV1 was seen as early as 2 weeks after initiation of treatment and was sustained through week 52 (Figure 5).
Figure 5 Mean Change (95% CI) from Baseline in Pre-Bronchodilator FEV1 (L) in NAVIGATOR
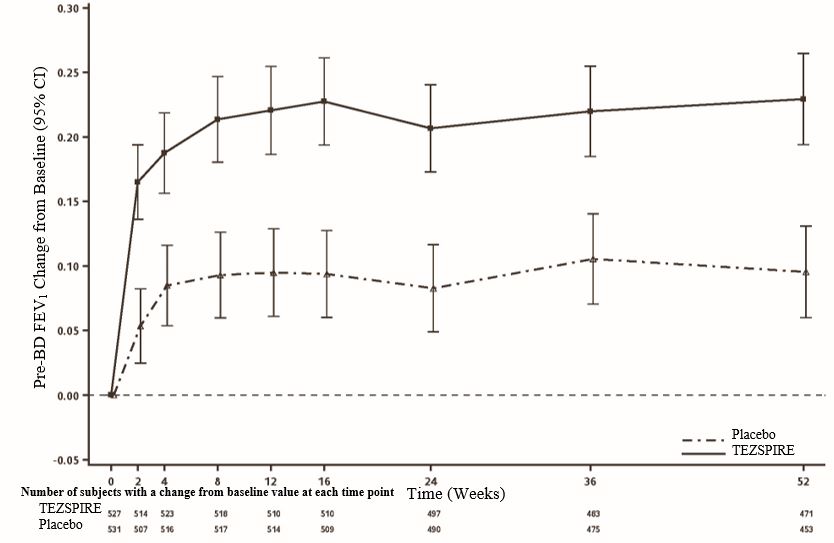
Patient Reported Outcomes
Changes from baseline in Asthma Control Questionnaire 6 (ACQ-6) and Standardized Asthma Quality of Life Questionnaire for ages 12 and older [AQLQ(S)+12] were also assessed as secondary endpoints in PATHWAY and NAVIGATOR. In both trials, more patients treated with TEZSPIRE compared to placebo had a clinically meaningful improvement in ACQ-6 and AQLQ(S)+12. Clinically meaningful improvement (responder rate) for both measures was defined as improvement in score of 0.5 or more at end of trial. In NAVIGATOR, the ACQ-6 responder rate for TEZSPIRE was 86% compared with 77% for placebo (OR=1.99; 95% CI 1.43, 2.76) and the AQLQ(S)+12 responder rate for TEZSPIRE was 78% compared with 72% for placebo (OR=1.36; 95% CI 1.02, 1.82). Similar findings were seen in PATHWAY.
Additional Trial
In a randomized, double-blind, parallel group, placebo-controlled clinical trial, the effect of TEZSPIRE (210 mg subcutaneously every 4 weeks) on reducing the use of maintenance OCS was evaluated. The trial enrolled 150 adult patients with severe asthma who required treatment with daily OCS (7.5 mg to 30 mg per day) in addition to regular use of high-dose ICS and a long- acting beta-agonist with or without additional controller(s). The primary endpoint was categorized percent reduction from baseline of the final OCS dose at Week 48 (≥90% reduction, ≥75% to <90% reduction, ≥50% to <75% reduction,
0% to <50 reduction, and no change or no decrease in OCS), while maintaining asthma control. TEZSPIRE did not demonstrate a statistically significant reduction in maintenance OCS dose compared with placebo (cumulative OR=1.28; 95% CI 0.69, 2.35).
SPL PATIENT PACKAGE INSERT SECTION
|
PATIENT INFORMATION TEZSPIRE**®**** (TEZ-SPY-ER)** (tezepelumab-ekko) injection, for subcutaneous use | ||
|
What is TEZSPIRE? TEZSPIRE is a prescription medicine used with other asthma medicines for the maintenance treatment of severe asthma in people 12 years of age and older whose asthma is not controlled with their current asthma medicine. TEZSPIRE helps prevent severe asthma attacks (exacerbations) and can improve your breathing. TEZSPIRE is not used to treat sudden breathing problems. Tell your healthcare provider if your asthma does not get better or if it gets worse after you start treatment with TEZSPIRE. It is not known if TEZSPIRE is safe and effective in children under 12 years of age. | ||
|
Do not use TEZSPIRE if you: • | ||
|
Before using TEZSPIRE, tell your healthcare provider about all of your medical conditions, including if you: • • • • • Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Do notchange or stop your corticosteroid medicines or other asthma medicines unless your healthcare provider tells you to. | ||
|
How should I use TEZSPIRE? • • • • • • • • | ||
|
What are the possible side effects of TEZSPIRE? TEZSPIRE may cause serious side effects, including: • | ||
|
∘ ∘ ∘ |
∘ ∘ ∘ | |
|
The most common side effects of TEZSPIRE include: | ||
|
• |
• |
• |
|
These are not all of the possible side effects of TEZSPIRE. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1‑800-FDA-1088. | ||
|
General information about the safe and effective use of TEZSPIRE Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use TEZSPIRE for a condition for which it was not prescribed. Do not give TEZSPIRE to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about TEZSPIRE that is written for health professionals. | ||
|
What are the ingredients in TEZSPIRE? Active ingredient: tezepelumab-ekko. Inactive ingredients: glacial acetic acid, L-proline, polysorbate 80, sodium hydroxide, and water for injection. | ||
|
Manufactured by: AstraZeneca AB, Sodertalje, Sweden SE-15185 US License No. 2059 At: Amgen Inc., One Amgen Center Drive, Thousand Oaks, CA 91320-1799 Marketed by: Amgen Inc. and AstraZeneca AB ©AstraZeneca and Amgen 2023 TEZSPIRE is a trademark of Amgen Inc. and AstraZeneca. For more information, go to https://www.TEZSPIRE.com or call 1-800-236-9933. | ||
|
This Patient Information has been approved by the U.S. Food and Drug Administration. Issued: 02/2023 |
|
INSTRUCTIONS FOR USE TEZSPIRE**®**** (TEZ-SPY-ER)** (tezepelumab-ekko) injection, for subcutaneous use Single-dose Pre-filled pen | |
|
This Instructions for Use contains information on how to inject TEZSPIRE. | |
|
Before using your TEZSPIRE pre-filled pen, your healthcare provider should show you or your caregiver how to use it the right way. Read this Instructions for Use before you start using your TEZSPIRE pre- filled pen and each time you get a refill. There may be new information. This information should not replace talking to your healthcare provider about your medical condition and your treatment. If you or your caregiver have any questions, talk to your healthcare provider. | |
|
Important information you need to know before injecting TEZSPIRE | |
|
Store TEZSPIRE in a refrigerator between 36°F to 46°F (2°C to 8°C) in its original carton until you are ready to use it. TEZSPIRE may be kept at room temperature between 68°F to 77˚F (20°C to 25°C) for a maximum of 30 days. Store TEZSPIRE in the original carton to protect it from light. When TEZSPIRE has reached room temperature,do not put it back in the refrigerator. Throw away (dispose of) TEZSPIRE that has been stored at room temperature for more than 30 days. | |
|
Do not use your TEZSPIRE pre-filled pen if: • • • • |
Do not: • • • . |
|
If any of the above happens, throw away the pre-filled pen in a puncture- resistant (sharps) container and use a new TEZSPIRE pre-filled pen. Each TEZSPIRE pre-filled pen contains 1 dose of TEZSPIRE that can only be used 1 time. TEZSPIRE is given only as an injection under the skin (subcutaneous). Keep the TEZSPIRE pre-filled pen and all medicines out of the sight and reach of children. | |
|
Your TEZSPIRE pre-filled pen | |
|
Do not remove the cap until Step 6 of these instructions when you are ready to inject TEZSPIRE. | |
|
 | |
|
Preparing to inject TEZSPIRE Step 1 – Gather supplies | |
|
• • • • • | |
|
| |
|
Step 2 – Prepare to use your TEZSPIRE pre-filled pen | |
|
Let TEZSPIRE come to room temperature between 68°F to 77˚F (20°C to 25°C) for about 60 minutes before giving the injection. Keep the pre-filled pen in its original carton to protect it from light. Do not warm the pre-filled pen in any other way. For example,do not warm it in a microwave or hot water, in direct sunlight, or near other heat sources. |
|
|
Do not put TEZSPIRE back in the refrigerator after it has reached room temperature. Throw away (dispose of) TEZSPIRE that has been stored at room temperature for more than 30 days. Do not remove the cap until Step 6. | |
|
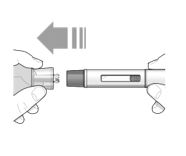
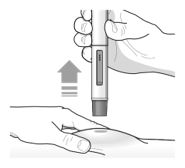
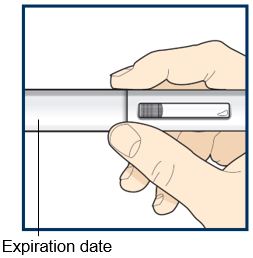
Step 3 – Remove and check the pre-filled pen | |
|
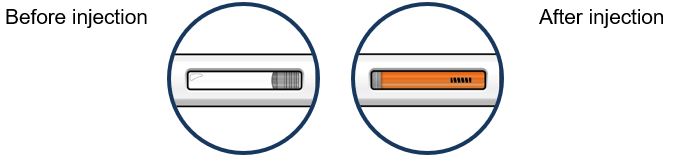
Grab the middle of the pre-filled pen body to remove the pre-filled pen from its tray. Check the pre-filled pen for damage. Do not use the pre-filled pen if the pre-filled pen is damaged. Check the expiration date on the pre-filled pen.Do not use the pre- filled pen if the expiration date has passed. Look at the liquid through the viewing window. The liquid should be clear and colorless to light yellow. Do not inject TEZSPIRE if the liquid is cloudy, discolored, or contains large particles. You may see small air bubbles in the liquid. This is normal. You do not need to do anything about it. |
|
|
Injecting TEZSPIRE Step 4 – Choose an injection site | |
|
If you are giving yourself the injection, therecommended injection site is the front of your thigh or the lower part of your stomach (abdomen).Do not inject yourself in the arm. A caregiver may inject you in the upper arm, thigh, or abdomen. For each injection, choose a different site that is at least 1 inch (3 cm) away from where you last injected. Do not inject: • • • • |
|
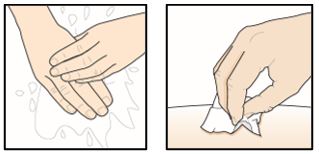
|
Step 5 – Wash your hands and clean the injection site Wash your hands well with soap and water. Clean the injection site with an alcohol wipe in a circular motion. Let it air dry. Do not touch the cleaned area before injecting. Do not fan or blow on the cleaned area. | |
|
| |
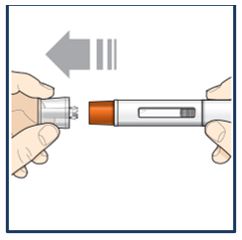
|
Step 6 – Pull off the cap | |
|
Do not remove the cap until you are ready to inject. Hold the pre-filled pen body with 1 hand, and carefully pull the cap straight off with your other hand. Put the cap to the side and throw it away later. The orange needle guard is now exposed. The orange needle guard is there to prevent you from touching the needle. Do not touch the needle or push on the orange needle guard with your finger. Do not put the cap back on the pre-filled pen. You could cause the injection to happen too soon or damage the needle. |
|
|
Step 7 – Inject TEZSPIRE | |
|
Follow your healthcare provider’s instruction on how to inject. You can either gently pinch the skin at the injection site or give the injection without pinching the skin. Inject TEZSPIRE by following the steps in figuresa, b, c andd. When injecting, you will hear the first click that tells you the injection has started. Press and hold the pre-filled pen for 15 seconds until you hear the second click. Do not change the position of the pre-filled pen after the injection has started. |
|
|
Position the pre-filled pen. • • | |
|
| |
|
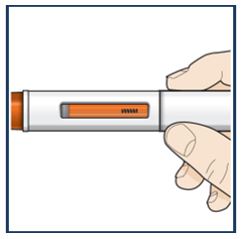
Step 8 – Check the viewing window Check the viewing window to make sure all the medicine has been injected. If the orange plunger rod does not fill the viewing window, you may not have received the full dose. If this happens or if you have any other concerns, contact your healthcare provider. | |
|
|
|
|
Step 9 – Check the injection site | |
|
There may be a small amount of blood or liquid where you injected. This is normal. Gently hold pressure over your skin with a cotton ball or gauze until the bleeding stops. Do not rub the injection site. If needed, cover the injection site with a small bandage. |
|
|
Disposing of TEZSPIRE Step 10 – Dispose of the used pre-filled pen safely | |
|
Each pre-filled pen contains a single dose of TEZSPIRE andcannot be used again.Do not put the cap back on the pre-filled pen. Put your used pre-filled pen and cap in asharps disposal container right away after use. Put other used supplies in your household trash. Do not throw away the pre-filled pen in your household trash. |
|
|
Throw away (Disposal) guidelines If you do not have a FDA-cleared sharps disposal container, you may use a household container that is: • • • • • When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the area that you live in, go to the FDA’s website at: [http://www.fda.gov/safesharpsdisposal](https://www.fda.gov/medical- devices/consumer-products/safely-using-sharps-needles-and-syringes-home-work- and-travel). Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container. Manufactured by: AstraZeneca AB, Sodertalje, Sweden SE-15185 US License No. 2059 At: Amgen Inc., One Amgen Center Drive, Thousand Oaks, CA 91320-1799 Marketed by: Amgen Inc. and AstraZeneca AB ©AstraZeneca and Amgen 2023 TEZSPIRE is a trademark of Amgen Inc. and AstraZeneca. For more information, go to https://www.TEZSPIRE.com or call 1-800-236-9933. | |
|
This Instructions for Use has been approved by the U.S. Food and Drug Administration. Issued: 02/2023 |