Parlodel
Parlodel Capsules and Tablets
fc2a08dd-4fb6-4ac4-9082-f99552fae25c
HUMAN PRESCRIPTION DRUG LABEL
Dec 15, 2023
Validus Pharmaceuticals LLC
DUNS: 801194619
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
bromocriptine mesylate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
bromocriptine mesylate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Drug Labeling Information
INDICATIONS & USAGE SECTION
INDICATIONS AND USAGE
Hyperprolactinemia**-Associated Dysfunctions**
Parlodel (bromocriptine mesylate) is indicated for the treatment of dysfunctions associated withhyperprolactinemia includingamenorrhea with or withoutgalactorrhea, infertility or hypogonadism. Parlodel treatment is indicated in patients withprolactin****-secreting adenomas, which may be the basic underlying endocrinopathy contributing to the above clinical presentations.Reduction intumor size has been demonstrated in both male and female patients with macroadenomas. In cases where adenectomy is elected, a course of Parlodel therapy may be used to reduce the tumor mass prior to surgery.
Acromegaly
Parlodel therapy is indicated in the treatment of acromegaly. Parlodel therapy, alone or as adjunctive therapy with pituitary irradiation or surgery, reduces serum growth hormone by 50% or more in approximately ½ of patients treated, although not usually to normal levels.
Since the effects of external pituitary radiation may not become maximal for several years, adjunctive therapy with Parlodel offers potential benefit before the effects of irradiation are manifested.
Parkinson’s Disease
Parlodel SnapTabs or capsules are indicated in the treatment of the signs and symptoms of idiopathic or postencephalitic Parkinson’s disease. As adjunctive treatment to levodopa (alone or with a peripheral decarboxylase inhibitor), Parlodel therapy may provide additional therapeutic benefits in those patients who are currently maintained on optimal dosages of levodopa, those who are beginning to deteriorate (develop tolerance) to levodopa therapy, and those who are experiencing “end of dose failure’’ on levodopa therapy. Parlodel therapy may permit a reduction of the maintenance dose of levodopa and, thus may ameliorate the occurrence and/or severity of adverse reactions associated with long-term levodopa therapy such as abnormal involuntary movements (e.g., dyskinesias) and the marked swings in motor function (“on-off” phenomenon). Continued efficacy of Parlodel therapy during treatment of more than 2 years has not been established.
Data are insufficient to evaluate potential benefit from treating newly diagnosed Parkinson’s disease with Parlodel. Studies have shown, however, significantly more adverse reactions (notably nausea, hallucinations, confusion and hypotension) in Parlodel-treated patients than in levodopa/carbidopa-treated patients. Patients unresponsive to levodopa are poor candidates for Parlodel therapy.
DESCRIPTION SECTION
DESCRIPTION
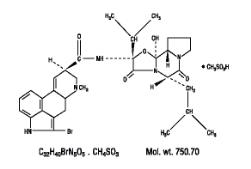
Parlodel® (bromocriptine mesylate) is an ergot derivative with potent dopamine receptor agonist activity. Each Parlodel® (bromocriptine mesylate) SnapTabs® tablet for oral administration contains 2½ mg and each capsule contains 5 mg bromocriptine (as the mesylate). Bromocriptine mesylate is chemically designated as Ergotaman-3′, 6′, 18-trione, 2-bromo-12′- hydroxy-2′- (1-methylethyl) -5′-(2-methylpropyl) -, (5′α) -monomethanesulfonate (salt).
The structural formula is:
2½ mg SnapTabs®
Active Ingredient: bromocriptine mesylate, USP
Inactive Ingredients: colloidal silicon dioxide, lactose, magnesium stearate, maleic acid, povidone, starch, and another ingredient
5 mg Capsules
Active Ingredient: bromocriptine mesylate, USP
Inactive Ingredients: colloidal silicon dioxide, gelatin, lactose, magnesium stearate, red iron oxide, silicon dioxide, sodium lauryl sulfate, starch, maleic acid, titanium dioxide, yellow iron oxide, and another ingredient
HOW SUPPLIED SECTION
HOW SUPPLIED
Parlodel (bromocriptine mesylate) SnapTabs
2½ mg
Parlodel is available in bottle containing 30 and 100 tablets of 2 ½ mg, each bottle contains a desiccant.
Round, off-white, bevelled-edge SnapTabs, each containing 2½ mg bromocriptine (as the mesylate). Engraved “PARLODEL 2½” on one side and “017” on the scored side. Complies with USP dissolution test 1.
Packages of 30………………………………………………. NDC 30698-202-30
Packages of 100………………………………………………NDC 30698-202-01
Parlodel (bromocriptine mesylate) Capsules
5 mg
Caramel and white capsules, each containing 5 mg bromocriptine (as the mesylate). Imprinted in red ink “PARLODEL 5 mg” on one half and “102” on other half.
Packages of 30………………………………………………. NDC 30698-201-30
Packages of 100………………………………………………NDC 30698-201-01
Store and Dispense
Store at 68º to 77ºF (20º to 25ºC); excursions permitted to 59º to 86ºF (15º to 30ºC) [See USP Controlled Room Temperature].
Dispense in a tight, light-resistant container.
Manufactured for and****Distributed by:
Validus Pharmaceuticals LLC
Parsippany, NJ 07054
info@validuspharma.com
www.validuspharma.com
1-866-982-5438
Product of Italy
© 2021 Validus Pharmaceuticals LLC
60004-05 July 2021
